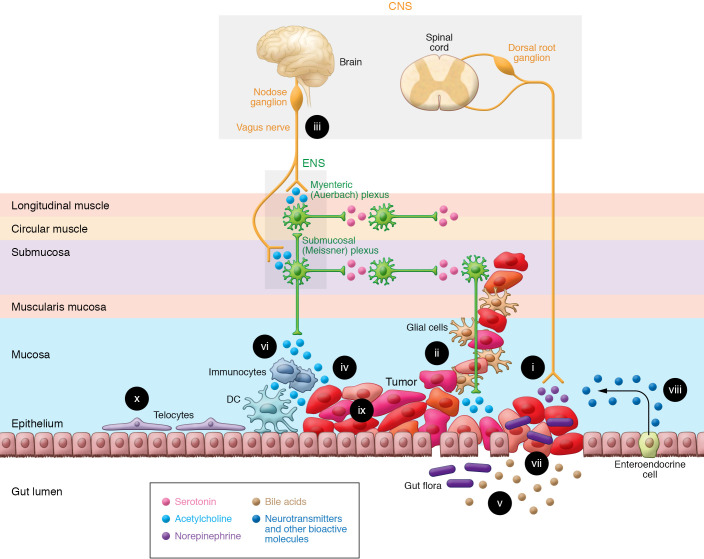
Figure 1. The GI neuron-cancer interface.
The ability of the gut-brain axis to modulate GI cancer progression is enhanced by the proximity and multidirectional crosstalk between numerous elements; these complex interactions provide opportunities for therapeutic intervention. (i) Cancer cells release nerve growth factors that promote neuronal tropism toward the tumor, enhancing access to neurotransmitters, metabolites, and the neural scaffold. Advanced cancer stages correlate with increased neural density. (ii) Perineural invasion, associated with worse outcomes, provides a path for tumor spread, access to neurotransmitters, and shielding from immune attack. (iii) Vagal innervation stimulates cancer progression by muscarinic mechanisms and modulates immune function. (iv) Neurotransmitters, like acetylcholine, produced and released by neurons, cancer cells, immunocytes, and possibly gut bacteria stimulate tumor growth, invasion, and dissemination. (v) Fecal bile acids, modified by gut bacteria, modulate immune and cancer cell function by several mechanisms, including activation of cancer cell muscarinic receptors. (vi) Immunocyte function is modulated by neurotransmitters released from the ENS, and cancer, immune, and enteroendocrine cells. (vii) Disruption of the intestinal barrier in the cancer field permits translocation of microorganisms that modulate immune and neural function. (viii) In response to bacterial and neural input, enteroendocrine cells, sprinkled throughout the mucosa, release neurotransmitters and other bioactive molecules. (ix) Cancer cells display intratumor heterogeneity and overexpress receptors for neurotransmitters and bioactive molecules. (x) Subepithelial telocytes are a critical source of pro-proliferative signaling for the intestinal stem cell niche; despite their prominent location, a functional role for telocytes at the neuron-cancer interface remains to be established.