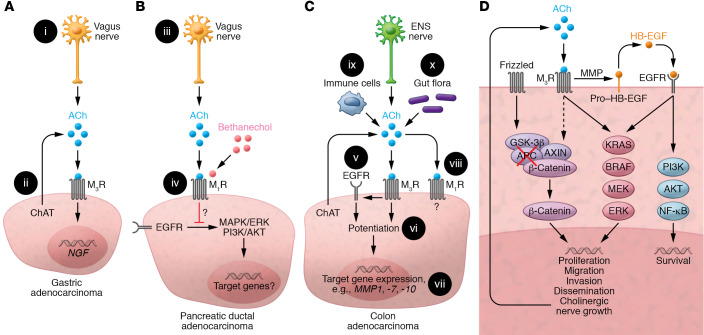
Figure 3. Muscarinic receptor activation in GI cancer.
(A) Gastric adenocarcinoma. (i) ACh release from vagal efferents activates M3 muscarinic receptors (M3R); vagotomy attenuates neoplasia. (ii) Cancer cells express ChAT, key for non-neuronal ACh synthesis; resulting ACh levels and their autocrine and paracrine impact on tumor progression remain uncertain. M3R activation induces nerve growth factor (NGF) expression. (B) PDAC. (iii) Treating mice with bethanechol, a non–subtype-selective muscarinic receptor agonist, activates muscarinic receptors. (iv) M1R activation attenuates PDAC progression by undefined mechanisms involving repressed EGFR signaling. (C) CRC. (v) M3R signaling transactivates EGFR; this is mediated by MMP7-mediated release of HB-EGF, an EGFR ligand. (vi) Concurrent activation of M3R and EGFR potentiates target gene expression. (vii) M3R activation selectively induces MMP1, MMP7, and MMP10 expression. MMP1 and MMP7 facilitate cell invasion. MMP7 also catalyzes the release of EGFR ligands (e.g., HB-EGF). (viii) M1R expression and activation attenuate colon cancer progression by unknown mechanisms. (ix) Immunocytes and (x) gut flora provide additional sources of non-neuronal ACh. (D) Post-M3R signaling alters gene expression and cancer cell function by impacting various signaling pathways. APC and/or β-catenin gene mutations free β-catenin from proteasomal destruction, promoting transcription of β-catenin target genes. M3R activation transactivates EGFR and augments β-catenin signaling. Resulting changes in downstream gene transcription stimulate cancer cell proliferation, survival, migration, invasion, and dissemination. Notably, induction of neurotrophin expression can promote neural growth and tropism, a feedback loop providing additional access to ACh and other neurotransmitters.