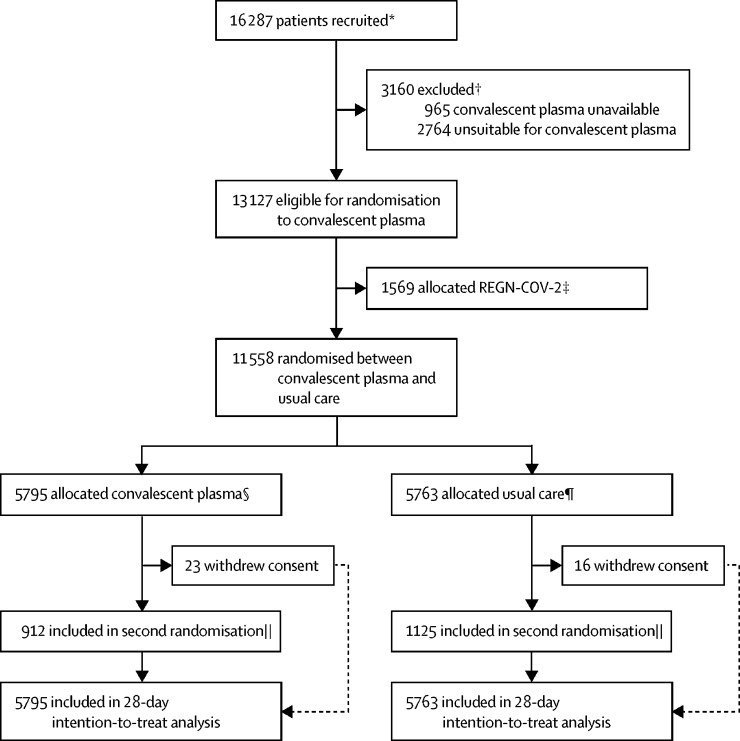
Figure 1.
Trial profile
*Number recruited overall during period that patients could be recruited into convalescent plasma comparison. †Reasons for exclusion are not mutually exclusive. ‡Patients in the group are not included in the analyses of this study. §5301 of 5795 patients with completed follow-up at time of analysis received convalescent plasma. ¶17 of 5763 patients with completed follow-up at time of analysis received convalescent plasma. ||A second randomisation to tocilizumab versus usual care in patients with hypoxia and C-reactive protein ≥75 mg/L was introduced in protocol version 4.0; 426 patients in the convalescent plasma group were randomly assigned to receive tocilizumab with 486 randomly assigned to receive usual care alone; 573 patients in the usual care group were randomly assigned to receive tocilizumab with 552 randomly assigned to receive usual care alone.