Abstract
The primary goal for any clinical trial after it receives a funding notification is to receive regulatory approval and initiate the trial for recruitment. Every trial must go through documentation and regulatory process before it can start recruiting participants and collecting data; this initial process of review and approval is known as the study start-up process (SSU). We evaluated the average time taken for studies to receive approvals. Using data from clinical trials conducted at the University of Kansas Medical Center, various times to reach the start of the study were calculated based on the dates of individual study. The results of this analysis showed that chart review studies and investigator-initiated trials had a shorter time to activation than other types of studies. Additionally, single-center studies had a shorter activation time than multi-center studies. The analysis also demonstrated that the overall processing time consistently had been reduced over time.
Keywords: Clinical trial activation, Institutional review board review
Highlights
-
•
The 2018 year’s trend shows reduced time to study start.
-
•
SSU process for non-cancer trial on an average requires four to six months.
-
•
The activation time of the SSU process varied for different study types and scopes.
1. Background
Throughout the study start-up process, there are a variety of obstacles that lengthen the time it takes for a study to be approved by IRB and open. David M. Dilts et al. delineated four types of administrative, or set-up, barriers: procedural, structural, infrastructural, and synchronicity [1,2]. The interactive effects of these barriers contribute to the increasing inefficiency of the process [3]. Researchers report that activation could require up to 110 individual steps performed by as many as 27 people [[1], [2], [3], [4]]. These steps constitute what is referred to as the study start-up process (SSU). There are many reasons for a study to have delays between each step within the SSU process [5,6]. Different strategies are being developed to eliminate inefficiencies in SSU [7]. By measuring the time it takes to move from one step to the next in the process, it is possible to identify possible bottlenecks. To optimize productivity, possible improvements can be made to eliminate or mitigate bottlenecks to improve the SSU process as a whole [8].
The Institutional Review Board (IRB) is crucial to the SSU process. Among other roles [9], the IRB is responsible for reviewing and approving documents, along with monitoring research that involves human subjects. A clinical trial must be approved by the IRB to ensure participants within the study are protected, and researchers are staying within set guidelines.
Clinical trials will often fall behind timelines due to delays throughout the SSU process. Some of these delays could spur from contract and budget negotiations with the sponsor, other internal reviews within the institution. The duration of the SSU process impacts the overall cost of the study. Characterizing how long it usually takes for a study to begin will help investigators and their sponsors estimate study costs and logistics. The primary focus of this analysis was to quantify the length of time for the completion of the SSU process. As for the secondary focus, the length of time for the different stages was examined across different types of trials.
2. Methods
At the University of Kansas Medical Center, the initiation of clinical trials goes through three stages with four events, as shown in Fig. 1, starting with the regulatory team organizing documents about the budget and contract following that would be trial registration with the IRB. The initial tasks working with the regulatory team and IRB involve gathering study relevant documents such as study protocols, consent form, and recruitment material as applicable. It is common for IRB to request revisions to these documents prior to approval.
Fig. 1.
SSU stages.
The first stage begins when the Principal Investigator (PI) is notified that the study will receive funding by their respective sponsor and ends when the PI submits relevant documentation to the IRB. Completing this stage initiates the start of the second stage, where the study gets submitted to the IRB to review the study from an ethical and participant safety perspective. The end of the second stage and the subsequent start of the third stage is marked by the IRB's approval of the study. The final stage, Stage 3, begins when the study is opened for recruiting participants. Since all the cancer trials must go through an additional ancillary review process, including those with the non-cancer would skew the results. Thus our team had decided to study cancer data separately in a follow-up study.
2.1. Data
The analysis was conducted for non-cancer clinical trials that were approved for funding between October 1st, 2012, a June 30th, 2018. By accessing data from the KUMC Clinical Trial Management System [10] and the electronic IRB system [9], time intervals between steps were measured for a total of 693 non-cancer studies. The data included the dates when each of the four SSU events took place. Four out of 693 studies had data with incorrect dates. Thus these four records were discarded from the analysis, possibly data entry error. The final dataset includes 689 non-cancer clinical trials.
2.2. Statistical analysis
Comparisons of hazard functions were conducted with the log-rank test. Modified Kaplan-Meier survival curves were used to show the difference in progression through the stages by study type, the study scope, and the starting year. Separate analysis for time to completion of each step was also conducted using the same methods. Significance level for all tests was set to α = 0.05. All analyses were performed using SAS 9.4. Copyright © [2002–2012] SAS Institute Inc.
3. Results
Across all studies, single-center studies go through the process 1.36 (1.16, 1.68) times quicker than multi-center studies. For single-center studies, the median time to activation is 157 days compared to multi-center studies, which is 214 days. Most studies spent most of the time getting through the first two stages. This could be due to the study feasibility process conducted by industrial/pharmaceutical partners.
An upset plot (Fig. 2) is used to show the aggregates of the various types of studies. The plot shows the majority of the studies performed at KUMC involve multiple centers. There were no single-center, industrial/pharmaceutical studies. Regardless of study type, the vast majority of the studies include multiple centers.
Fig. 2.
Upset plot of the various types of studies and scopes.
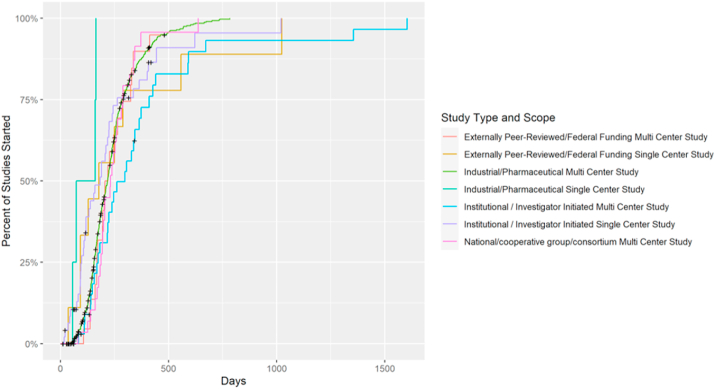
Multi-center investigator-initiated trials (IITs) took longer than single-center IITs to complete the full SSU process (p = 0.0002, Fig. 3) with median times of 298 and 183 days respectively. Out of the 689 studies, 91 (13%) did not complete the SSU process with 68 of those studies being multi-centered, industrial/pharmaceutical. Focusing on those specific multi-centered, industrial/pharmaceutical studies, 41 did not complete stage two with 27 not finishing stage three.
Fig. 3.
SSU across study type and scope.
There is a significant trend (p < 0.0001) shown in the curves that show what year the study initially received funding. A year to year trend depicting the studies completing the SSU process at a quicker rate (Fig. 4). To further investigate the factors behind the faster pace over the years, the three stages were examined across these years. When examining the three different stages, studies from more recent years are completing the first two stages faster than studies that received funding prior to 2018. However, there are no significant differences in the curves when examining the third stage.
Fig. 4.
SSU across starting the year.
The number of participating sites associated with a study impacts the overall SSU timeline. The activation time for different study scopes was shown to have a significant difference in the activation time. Across all study types, the median time to activation for single-center studies was 157 as compared to about 214 for multi-center studies. Looking at Table 1, it can also be seen that there is a considerable amount of time spent going through the first interval. Multi-center research could expect to spend sixty to ninety days going through the first stage.
Table 1.
SSU metrics.
| Study Type | Single Center |
|||
|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Full SSU | |
| Industrial/Pharmaceutical | 36 [34, 56] | 37 [29, 42] | 10 [2, 34] | 117 [69, 162] |
| National/cooperative group/consortium | NA | NA | NA | NA |
| Institutional/Investigator Initiated | 61 [26, 107] | 45 [34, 62] | 22 [1, 66] | 154 [95, 233] |
| Externally Peer-Reviewed/Federal Funding | 30 [14, 101] | 38 [27, 54] | 21 [0, 135] | 178 [93, 289] |
| All | 54 [23, 107] | 42 [32, 61] | 19 [0, 69] | 157 [93, 241] |
|
Multi-Center |
||||
| Stage 1 | Stage 2 | Stage 3 | Full SSU | |
| Industrial/Pharmaceutical | 100 [63, 150] | 59 [41, 85] | 28 [12, 54] | 209 [153, 275] |
| National/cooperative group/consortium | 125 [92, 153] | 59 [44, 86] | 34 [12, 80] | 230 [185, 287] |
| Institutional/Investigator Initiated | 100 [70, 152] | 67 [47, 110] | 44 [17, 88] | 261 [172, 392] |
| Externally Peer-Reviewed/Federal Funding | 100 [72, 155] | 61 [39, 80] | 43 [29, 80] | 204 [169, 270] |
| All | 103 [63, 151] | 60 [41, 86] | 29 [13, 57] | 214 [156, 280] |
|
Both |
||||
| Stage 1 | Stage 2 | Stage 3 | Full SSU | |
| Industrial/Pharmaceutical | 100 [62, 149] | 55 [35, 81] | 23 [6, 49] | 196 [143,268] |
| National/cooperative group/consortium | 125 [92, 153] | 59 [44, 86] | 34 [12, 80] | 230 [185, 287] |
| Institutional/Investigator Initiated | 79 [40, 134] | 48 [34, 75] | 18 [0,63] | 183 [98, 337] |
| Externally Peer-Reviewed/Federal Funding | 84 [37, 140] | 44 [36, 77] | 36 [15, 82] | 206 [165, 288] |
| All | 98 [59, 149] | 55 [35, 80] | 24 [5, 52] | 199 [141, 274] |
Each cell shows the median time in days with the first and third quartile for each stage.
4. Discussion
There is a current trend of studies getting through the SSU process at a faster rate over the last few years. Out of the 689 studies, roughly 88% of the studies complete the process and initiate enrollment. There are many reasons why studies do not make through the SSU process. Some of the reasons relate to budgeting and/or contract terms. At times, when a study team submits documentation for IRB review, the IRB will ask the investigator to make a modification to their study. Other times, a sponsor might ask the investigator to modify their study. The investigator might decide to forego the study. These delays seem common in industrial/pharmaceutical studies done across multiple centers. With multi-site studies, the complexity of interacting with the collaborating sites creates a complicated and time-consuming SSU process. Most studies will experience some sort of delay, whether it is because of peer review issues or discussions with the sponsor about contract agreements. Any study sponsored by or funded by a pharma/biotech company will have contracts and budgets to negotiate, which require a great deal of time before all parties meet agreeable terms. It is to also note that KUMC was the lead site on very few studies in the study population. Open ended questions were given to roughly 30 non-cancer trialists on different studies as part of an evaluation for Frontiers and Greater Plains Collaborative. Their responses to the question “What have been the most frequent barriers you have faced that have delayed project start up?” were used to help gain more insight on the reasons behind any delays they experienced on their respective study. Examining the responses, one of the most common sentiment was – “having to wait months to hear back from either their sponsor to make modifications to certain documents” (these are typically private conversations) or adapting to the unfamiliar institutional process.
A couple specific examples within the study population showed a need for resubmission and requests for certain documents to clarify their protocols. On some occasions, it took months for the study team to submit request documents.
One of the limitations with our study has been the exclusion of the cancer trials; All the cancer trials must go through another set of ancillary reviews before they could be submitted to IRB review. Thus, the time it takes to reach the activation step is usually longer for cancer clinical trials. Describing the SSU process for cancer trials will be future work. It should also be noted that less than a handful of the studies in the study population were three chart reviews, which tend to have an expedited total start up time and possibly biasing the estimates. Due to the small amount of these specific studies, we do not see them to have a significant effect on the estimates.
5. Conclusion
Our study has attempted to perform a descriptive statistical analysis around the SSU process for all the non-cancer studies which are conducted at KUMC. Furthermore, the outcomes of our study demonstrated that the SSU process for non-cancer trials required approximately 120–180 days from the site selection/receipt of study documents date to actual study activation. Additionally, the activation time of the SSU process varied for different research types and across different scopes. Overall, we noted a trend where the SSU process has been quicker and more efficient over time. It is unsure as to why this has been the case, as more information and data would need to be gathered to examine possible confounding variables. Some of the recent initiatives have been the real reason behind the reduced start-up times. Those initiatives include – PI (Principle Investigator) and Research Coordinator Bootcamp, End-user manual and resource library, modernizing the application process, additional IRB committee members being added to streamline the review process, and intra-campus collaboration among various stakeholders. Every month our regulatory body conducts a bootcamp focused towards what the procedures are and resources that are available and how adhering to the procedures would expedite the review process. Using these start up times, along with each interval time estimate, could be helpful in gauging study start up length and can be compared to other medical and educational centers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dilts D.M., Sandler A.B. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J. Clin. Orthod. 2006;24:4545–4552. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]
- 2.Dilts D.M., Sandler A., Cheng S., Crites J., Ferranti L., Wu A., Gray R., MacDonald J., Marinucci D., Comis R. Development of clinical trials in a cooperative group setting: the eastern cooperative oncology group. Clin. Canc. Res. 2008;14:3427–3433. doi: 10.1158/1078-0432.CCR-07-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silberman G., Kahn L.K. Burdens on research imposed by institutional review boards: the state of the evidence and its implications for regulatory reform. Milbank Q. 2011 doi: 10.1111/j.1468-0009.2011.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dilts D.M., Sandler A.B., Baker M., Cheng S.K., George S.L., Karas K.S., McGuire S., Menon G.S., Reusch J., Sawyer D., Scoggins M., Wu A., Zhou K., Schilsky R.L. Processes to activate phase III clinical trials in a cooperative oncology group: the case of cancer and leukemia group B. J. Clin. Orthod. 2006;24:4553–4557. doi: 10.1200/JCO.2006.06.7819. [DOI] [PubMed] [Google Scholar]
- 5.Lamberti Mary Jo. Clinical trials take a long time to get started. Here's how to speed it up. 2017. https://www.statnews.com/2018/03/28/clinical-trials-startup-speed/ [WWW Document]. STAT. URL. accessed 3.19.20.
- 6.Bast Robert C. Project zero delay accelerates drug's path to clinical trial. 2009. https://www.newswise.com/articles/project-zero-delay-accelerates-drugs-path-to-clinical-trial [WWW Document]. Newswise. URL. accessed 3.19.20.
- 7.Martinez D.A., Tsalatsanis A., Yalcin A., Zayas-Castro J.L., Djulbegovic B. Activating clinical trials: a process improvement approach. Trials. 2016;17:106. doi: 10.1186/s13063-016-1227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilts D.M., Sandler A.B., Cheng S.K., Crites J.S., Ferranti L.B., Wu A.Y., Finnigan S., Friedman S., Mooney M., Abrams J. Steps and time to process clinical trials at the cancer therapy evaluation program. J. Clin. Orthod. 2009;27:1761–1766. doi: 10.1200/JCO.2008.19.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Description of institutional review board of university of Kansas. 2018. http://www.kumc.edu/compliance/human-research-protection-program/institutional-review-board.html Medical Center [WWW Document] accessed 3.19.20.
- 10.Clinical Trial Management System Department of biostatistics & data science. http://www.kumc.edu/school-of-medicine/department-of-biostatistics-and-data-science/clinical-trial-and-data-coordination-section.html