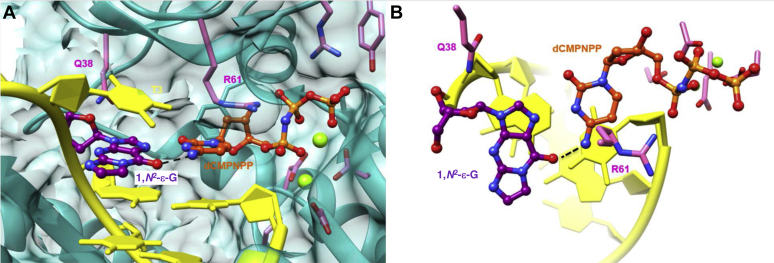
Figure 8.
Active site conformation in the ternary hpol η insertion step complex with dCMPnPP opposite 1,N2-ε−G in the 5′-T(εG)A-3′ template sequence context (oligonucleotide 1).A, view into the DNA major groove. B, rotated by 90° and viewed perpendicular to the cytosine and adduct base planes. Selected active site residues are colored by atom with carbon atoms shown in purple (1,N2-ε−G), orange (incoming dCMPnPP), or pink (Arg-61 and Gln-38 from the finger domain and Asp/Glu coordinating Mg2+ ions that are shown as light green spheres). The remaining template and primer residues are colored in yellow and H-bonds involving the incoming nucleotide are drawn with dashed lines. It is unlikely that N1 of 1,N2-ε−G and N3 of dCMPnPP are H-bonded because the pH of the crystallization solution is too high for cytosine to be protonated at N3 and a tautomeric form of the adduct with the hydrogen on N1 cannot be invoked.