Fig. 2. Assignment of HN peaks of amino-acid main chain of labeled Neh2.
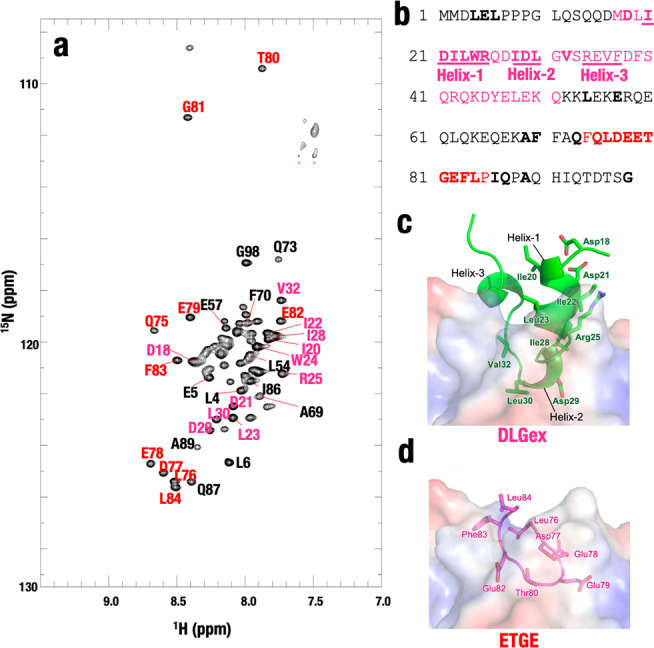
a 2D TROSY-HSQC spectrum shows HN peaks of the main chain of amino acids of labeled Neh2 domain protein. b Alignment of mouse Neh2 domain. The assigned amino acids are in bold. The amino acids in Helix-1, Helix-2, and Helix-3 in the DLGex motif are underlined. c, d Structure of DLGex (c) and ETGE (d) motifs associating with the Keap1 pocket. Note that the DLGex motif possesses three-helix regions (Helix-1, 19–25; Helix-2, 28–30; Helix-3, 34–37) (PDB 3WN7, ref. 8), whereas the ETGE motif forms a β-hairpin structure (PDB 2DYH, ref. 16). We have proposed the two-site binding model in which each motif binds to a similar pocket structure in the Keap1-DC domain of the Keap1 homodimer (as shown in Fig. 1a).
