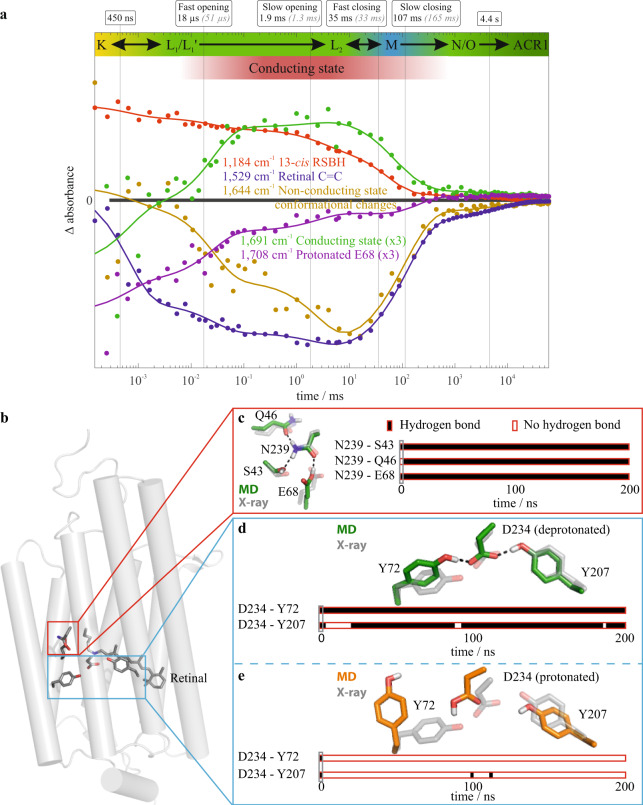
Fig. 2. Time courses of marker absorption bands of GtACR1.
a Merged time-resolved data obtained from step-scan and rapid-scan FTIR difference spectroscopic measurements. The conducting state assignment is based on the half-lives obtained in the spectroscopic global fit analysis (bold black numbers and vertical solid black lines) and the published electrophysiologically determined half-lives of channel opening and closing32 (gray italic numbers). The absorbance key bands were derived from the amplitude spectra (Supplementary Fig. 2). The 1644 cm−1 band (ocher) reflects conformational changes during channel opening and closing in the amide I region. The 1691 cm−1 band (green) corresponds to channel opening and serves as a marker band for the conducting state. The 1184 cm−1 band (red) reflects the protonated 13-cis retinal previously established in microbial rhodopsins37. The 1529 cm−1 band (blue) reflects the retinal C=C vibrations in the ground state39. Further kinetics are shown in Supplementary Fig 3. Based on these IR spectroscopic data along with UV/VIS data (Supplementary Fig. 3), we derived an extended GtACR1 photocycle model with a non-conducting L1 and an opened L2 state shown in Fig. 4. For a detailed discussion of all kinetic information, please refer to Supplementary Figs. 2–4 and 6 and Supplementary Note 1. b Overview of the GtACR1 ground state structure based on monomer A of protein data bank (PDB)-ID 6CSM26. c Representative hydrogen bond network of the central constriction site for protonated Glu-68 obtained using a molecular dynamics (MD) simulation initiated by monomer A of the X-ray structure 6CSM. d Representative structure (green) and contact analysis of a 100 ns trajectory initiated by monomer A of the X-ray structure 6CSM with deprotonated Asp-234 and Glu-68. The protonated SB is compared to the X-ray structure (gray). e Representative structure (orange) and contact analysis of a 100 ns MD simulation trajectory as described in (d) but with protonated Asp-234. The source data for a, c–e is given in Supplementary Data 1.