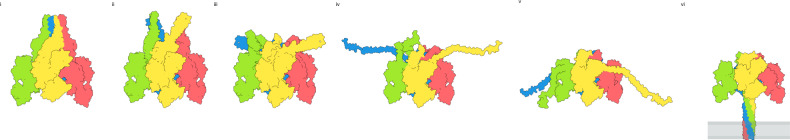
Fig. 5. Proposed model of Vip3Bc1 conformational rearrangement upon processing.
Cleavage at K205 liberates helices α1-α4 allowing dissociation of inter-subunit interactions at the tip of the complex (i), the helices of domains 2 that are C-terminal to the cut site (from α5 onwards), remain in essentially their original position stabilising the central interface of the tetramer. The α4 helices form a central helical bundle while the other helices N-terminal to the cut site (α1-3) are able to unfurl and move down between the propeller domains to form a new helical bundle (ii–v), which interacts with the membrane via the ends of the helical bundle (vi).