Abstract
Throughout North America, Dermacentor spp. ticks are often found feeding on animals and humans, and are known to transmit pathogens, including the Rocky Mountain spotted fever agent. To better define the identity and distribution of Dermacentor spp. removed from dogs and cats in the United States, ticks submitted from 1,457 dogs (n = 2,924 ticks) and 137 cats (n = 209 ticks) from veterinary practices in 44/50 states from February 2018-January 2020 were identified morphologically (n = 3,133); the identity of ticks from regions where Dermacentor andersoni (Stiles) have been reported, and a subset of ticks from other regions, were confirmed molecularly through amplification and sequencing of the ITS2 region and a 16S rRNA gene fragment. Of the ticks submitted, 99.3% (3,112/3,133) were Dermacentor variabilis (Say), 0.4% (12/3,133) were D. andersoni, and 0.3% (9/3,133) were Dermacentor albipictus (Packard). While translocation of pets prior to tick removal cannot be discounted, the majority (106/122; 87%) of Dermacentor spp. ticks removed from dogs and cats in six Rocky Mountain states (Montana, Idaho, Wyoming, Nevada, Utah, and Colorado) were D. variabilis, suggesting this species may be more widespread in the western United States than is currently recognized, or that D. andersoni, if still common in the region, preferentially feeds on hosts other than dogs and cats. Together, these data support the interpretation that D. variabilis is the predominant Dermacentor species found on pets throughout the United States, a finding that may reflect recent shifts in tick distribution.
Keywords: American dog tick, dog, cat, Dermacentor
Graphical Abstract
Dermacentor spp. are some of the most common ticks found on dogs and cats in North America (Dryden and Payne 2004, Thomas et al. 2016, Little et al. 2018, Saleh et al. 2019). A national survey of pets across the United States showed that, of pets infested with ticks, 17.9% of cats and 35.6% of dogs are infested with D. variabilis (Saleh et al. 2019). In addition to being a primary parasite, D. variabilis can cause tick paralysis in pets and people, is an important vector of Rickettsia rickettsii and Francisella tularensis, and has been implicated as a primary or secondary vector associated with transmission of several other tick-borne pathogens (Dryden and Payne 2004, Jongejan and Uilenberg 2004). Historically, D. variabilis was thought to be present primarily in southcentral and southeastern Canada, the eastern half of the United States, and in Mexico, with a second population found along the West Coast, but was considered largely absent from the Rocky Mountain region (Bishop and Trembley 1945, Dryden and Payne 2004, Dergousoff et al. 2013, Guzmán-Cornejo et al. 2016, CDC 2020). Instead, a related species (D. andersoni), appropriately called the Rocky Mountain wood tick, was widely thought to predominate in this higher elevation area of the continent (Dryden and Payne 2004, CDC 2020).
In recent years, the geographic distributions of several tick species in North America have expanded due to a variety of factors, such as climate change and habitat change, that have made new areas supportive of tick populations. Human and animal-mediated introductions then facilitate expansion into these regions (Little 2013, MacDonald 2018, Sonenshine 2018). Northward and westward expansion of D. variabilis is well documented in Canada, and recent models indicate habitat suitability for this tick is expected to increase dramatically in those regions in future decades (Dergousoff et al. 2013, Yunik et al. 2015, Wood et al. 2016, Minigan et al. 2018). A similar shift may be occurring in the Rocky Mountain region of the United States, but recognizing the change could be delayed by morphologic similarities between D. variabilis and D. andersoni (Dryden and Payne 2004, Dergousoff and Chilton 2007). Some recent publications have identified Dermacentor species from the Rocky Mountain region by ‘incorporat[ing] information on geographic range to allow species identification,’ an approach that may lead to confusion about the current distribution of a given species, particularly given that the importance of D. variabilis is thought to be under-recognized in some areas (Nieto et al. 2018, Lehane et al. 2020). The aim of the present study was to confirm the identity of Dermacentor spp. ticks removed from dogs and cats across the United States, with a particular focus on Dermacentor spp. submitted from the western and central regions, to more accurately confirm the current geographic distribution and host preferences of members of this genus.
Materials and Methods
Ticks used in the current study were obtained through an ongoing national survey of ticks on pets as previously described (showusyourticks.org; Saleh et al. 2019) following collection and submission to Oklahoma State University by veterinarians across the United States. Ticks were collected from February 2018 to January 2020 and include those collected in 2018 and published in an earlier report (Saleh et al. 2019) and a second year of ticks collected in 2019 and reported in the present paper for the first time. Ticks were submitted together with information about pet species, age, weight, estimated percent time spent outside as reported by the owner, sex, and spay/neuter status. Upon receipt, all ticks were identified to species using standard morphologic keys and then stored in 70% ethanol at −20°C until further work was performed (USDA 1976, Lindquist et al. 2016).
The identity of all Dermacentor spp. ticks from the western United States (n = 188), all Dermacentor spp. ticks from states bordering the western United States (n = 122), and a subset of remaining ticks from the Midwest (n = 163), South (n = 147), and Northeast (n = 42) United States were confirmed molecularly. To achieve a broadly representative subsampling, up to 13 ticks (if available) were randomly selected for molecular confirmation from each state. Molecular confirmation was made using two nucleic acid targets, namely, fragments of the mitochondrial 16S rRNA gene and the nuclear ribosomal second internal transcribed spacer (ITS-2), as previously described (Dergousoff and Chilton 2007, Nadolny et al. 2011). Nucleic acid was extracted with a commercial kit (Illustra GenomicPrep Kit, GE Healthcare) and polymerase chain reaction (PCR) was used to amplify the two genetic markers. Following confirmation of amplification on a 2% agarose gel, products were column-purified and sequenced directly with an ABI 3730 capillary sequencer (Applied Biosystems, Foster City, CA) at the Oklahoma State University Molecular Core Facility (Stillwater, OK). Upon verification of high-quality electropherograms by visual inspection and routine editing, sequences were compared to those published for Dermacentor spp. and to all available sequences in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Descriptive statistics (mean, range, proportion, and exact binomial 95% CI were calculated with Microsoft Excel (Microsoft Office Professional Plus 2016). Geographic regions used in comparisons were determined as previously described (Blagburn et al. 1996).
Results
A total of 3,133 Dermacentor spp. were examined, including 2,924 collected from 1,457 dogs and 209 from 137 cats. Age, weight, estimated time outside, sex, and spay/neuter status of dogs and cats infested with Dermacentor spp. are summarized in Table 1. An average of 1.5 and 1.2 ticks (median = 1) were collected from each dog and cat, respectively, with as many as 23 or 4 Dermacentor spp. on a single dog or cat, respectively.
Table 1.
Age, weight, estimated time spent outside, and sex and spay/neuter status for dogs and cats infested with Dermacentor spp. from across the United States
| Dogs | Cats | |
|---|---|---|
| Age (yr), mean (range) | 5.3 (0.1–19) 95% CI 5.1–5.5 |
5.4 (0.1–18) 95% CI 4.6–6.2 |
| Weight (kg), mean (range) | 21.1 (0.2–90.9) 95% CI 20.3–21.8 |
4.2 (0.2–11.4) 95% CI 3.8–4.5 |
| Estimated time outside (%), mean (range) | 44.0 (0–100) 95% CI 42.1–45.9 |
73.0 (0–100) 95% CI 65.7–80.2 |
| Total female (n, %) | 660 (46.5%) 95% CI 43.9–49.1 |
56 (42.8%) 95% CI 34.6–51.3 |
| Female intact (n, %) | 203 (30.8%) 95% CI 27.4–34.4 |
22 (39.3%) 95% CI 27.6–52.4 |
| Female spayed (n, %) | 457 (69.2%) 95% CI 65.6–72.7 |
34 (60.7%) 95% CI 47.6–72.4 |
| Total male (n, %) | 759 (53.5%) 95% CI 51.0–56.1 |
75 (57.3%) 95% CI 48.7–65.4 |
| Male intact (n, %) | 280 (36.9%) 95% CI 33.5–40.4 |
26 (34.7%) 95% CI 24.9–46.0 |
| Male neutered (n, %) | 479 (63.1%) 95% CI 59.6–66.5 |
49 (65.3%) 95% CI 54.0–75.1 |
kg, kilograms.
Morphologic identification confirmed the identity of ticks as 3,112 D. variabilis (99.3%, 95% CI 99.0–99.6), 12 D. andersoni (0.4%, 95% CI 0.2–0.7), and 9 D. albipictus (0.3%, 95% CI 0.1–0.6). Molecular identification using two genetic markers on a subset (n = 662) confirmed the identity of 641 D. variabilis, 12 D. andersoni, and 9 D. albipictus. Of the submissions that included state of origin on the pet information form, the Dermacentor spp. primarily originated from the Midwest (n = 1,660; 53.0%), followed by the South (n = 853; 27.2%), the Northeast (n = 430; 13.7%), and the West (n = 188; 6.0%); two ticks were submitted without a state of origin. Only D. variabilis were submitted from the Northeast; both D. variabilis and D. albipictus were received from the South and Midwest; and D. variabilis, D. andersoni, and D. albipictus were submitted from the West (Table 2).
Table 2.
Number of Dermacentor variabilis and Dermacentor andersoni adult and nymphal ticks collected from dogs and cats by species, stage, and geographic region
| Species | Stage | Region | |||
|---|---|---|---|---|---|
| Northeast | South | Midwest | West | ||
| Dermacentor variabilis | Female | 284 | 493 | 964 | 103 |
| Male | 146 | 359 | 689 | 62 | |
| Nymph | 0 | 0 | 5 | 5 | |
| Dermacentor andersoni | Female | 0 | 0 | 0 | 8 |
| Male | 0 | 0 | 0 | 4 | |
| Nymph | 0 | 0 | 0 | 0 | |
| Dermacentor albipictus | Female | 0 | 1 | 1 | 1 |
| (Duncan et al. 2020) | Male | 0 | 0 | 0 | 1 |
| Nymph | 0 | 0 | 1 | 4 | |
| Total | 430 | 853 | 1,660 | 188 |
No Dermacentor spp. larvae were submitted from pets over the 2-yr period. The Dermacentor albipictus listed here were reported in an earlier, multi-institutional paper (Duncan et al. 2020).
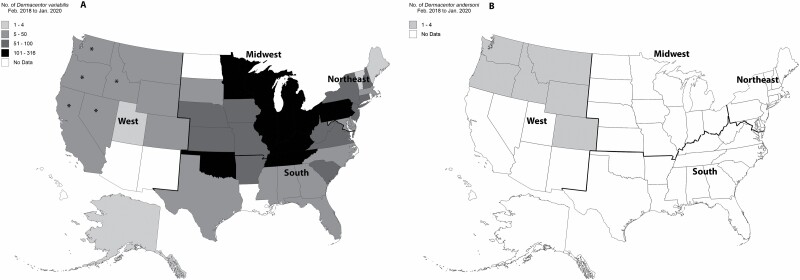
Every state with Dermacentor spp. submitted (n = 44 states) had D. variabilis collected from pets (Fig. 1A); D. andersoni was submitted from only six western states (Montana, Colorado, Idaho, Wyoming, Oregon, and Washington) and in lower numbers (Fig. 1B). Out of the 188 ticks submitted from the West, the majority (90.4%, 170/188, 95% CI 85.3–93.9) were D. variabilis. More specifically, 106 of the 122 ticks (86.9%, 95% CI 79.7–91.9) submitted from six Rocky Mountain states (Montana, Idaho, Wyoming, Nevada, Utah, and Colorado) were D. variabilis while D. andersoni accounted for 10 out of 122 ticks (8.2%, 95% CI 4.4–14.6) submitted from these six states. Sequence analysis (16S rRNA gene) revealed D. variabilis submitted from California, Oregon, Washington, Idaho, and Nevada were most closely related (~99.5–100% similarity) to D. variabilis from California, or the ‘western’ D. variabilis population (GenBank Accession MG834237 and MG834234). Sequence of all other submitted D. variabilis from the western United States (Colorado, Utah, Wyoming, Montana) were identical (100%) to that of D. variabilis from Colorado and Indiana, or the ‘eastern’ D. variabilis population (GenBank Accession MG834244 and MG834241).
Fig. 1.
Number of Dermacentor variabilis (A) and Dermacentor andersoni (B) collected from dogs and cats in different states in the United States, February 2018 to January 2020. Identification was confirmed molecularly for all ticks in the western United States and for a representative subset of ticks in other regions. Analysis showed distinct sequences indicating ‘western’ D. variabilis only in the five western states denoted with an asterisk (*); all other D. variabilis sequences were identical to those previously reported from the ‘eastern’ populations of D. variabilis.
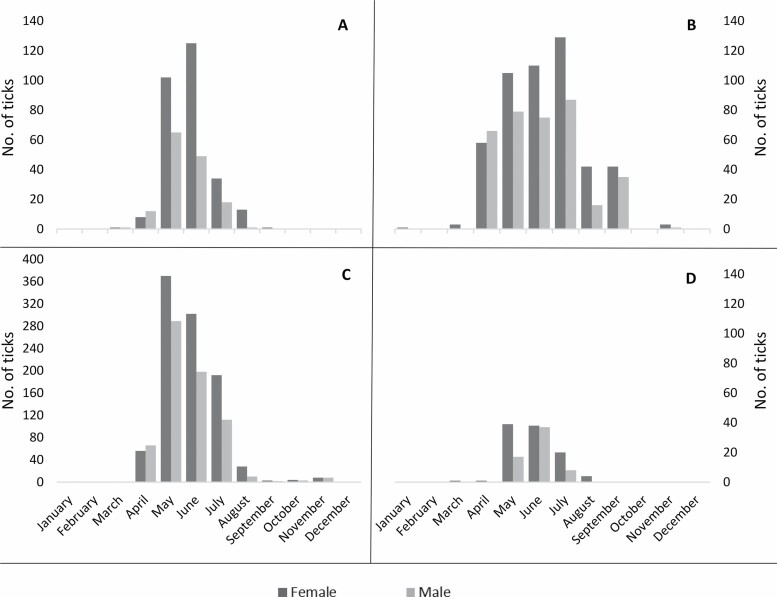
Adult D. variabilis was the stage of Dermacentor most often submitted (n = 3,102), and the time of collection peaked in the warmer, summer months in every region (Fig. 2). In the Northeast, Midwest, and West, tick collection was highest in May and June but collections declined in July, and reached almost zero by September (Fig. 2A, C, and D). In the South, tick collection increased in May, remained high through July, and then numbers declined in August, reaching zero by October (Fig. 2B). More female ticks were collected than male (n = 1,846 (59.5%) and n = 1,256 (40.5%), respectively), and the peak time of collection for each stage was similar for each region. However, in the Northeast, South, and Midwest, more male ticks than female ticks were collected in April just prior to the peak of female ticks in the summer months (Fig. 2).
Fig. 2.
Month of collection of adult Dermacentor variabilis from dogs and cats in the Northeast (A), South (B), Midwest (C), and West (D) regions of the United States. Due to the large number of Dermacentor variabilis ticks submitted from the Midwest, the scale of the y-axis for Fig. 2C is adjusted.
Discussion
Dermacentor variabilis was the predominant Dermacentor species recovered from dogs and cats in every region in the present study. In each of the 44 states from which Dermacentor spp. ticks were submitted, including those in the Rocky Mountain region, more D. variabilis were submitted than D. andersoni as confirmed both morphologically and molecularly. Indeed, 10-fold more D. variabilis than D. andersoni were submitted from dogs and cats examined by veterinarians in Rocky Mountain states, a finding that is surprising in light of current distribution maps for these two species (CDC 2020). A few D. albipictus were also identified and are reported elsewhere (Duncan et al. 2020). Of the 1,594 dogs and cats with Dermacentor spp. included in the present study, 1,330 (83.4%) were only infested with a single species of Dermacentor; D. variabilis was not found co-infesting any pets with other Dermacentor spp., although 264 animals (16.6%) had a co-infestation with ticks of a different genus, including Amblyomma americanum (59.1%), Ixodes scapularis (30.3%), Amblyomma maculatum (6.8%), Rhipicephalus sanguineus (2.3%), Haemaphysalis longicornis (1.1%), and I. pacificus (0.4%; data not shown).
Translocation is sometimes found to account for unexpected geographic distribution of ticks removed from pets or people that have recently traveled (Jones et al. 2017, Xu et al. 2019). However, in the present study, sequence analysis revealed that every D. variabilis collected from the far western United States was placed in the ‘western D. variabilis’ phylogenetic group, and all D. variabilis from the eastern side of the Rocky Mountain range were ‘eastern D. variabilis,’ suggesting geographic expansion of the eastern population is more likely, and that, to the extent that translocation of D. variabilis on pets occurs, it is more common within regions rather than between regions. A shift in the distribution of D. andersoni and D. variabilis has been suggested by others and indicates that, should the findings in the present paper be confirmed by direct collection of questing ticks in the region, tick distribution maps for Dermacentor spp. may benefit from reconsideration and updating (James et al. 2015, Minigan et al. 2018, CDC 2020). Alternatively, D. andersoni may still be present and abundant in the Rocky Mountain region but sympatric with D. variabilis, and the latter species preferentially feeds on the hosts—dogs and cats—surveyed in the present paper. Host preferences for the different Dermacentor spp. have been reported although both D. variabilis and D. andersoni readily feed on pets (Cooley 1938, Dryden and Payne 2004).
Our data demonstrate the distribution of D. andersoni and D. variabilis likely overlaps over a much larger area than originally reported. Historically, sympatric populations of these two species were known to occur in the western Great Plains region of the United States and in southern Saskatchewan (Gibbons 1939, Bishop and Trembley 1945, Dergousoff et al. 2013), with D. andersoni reportedly more common in areas with hot and dry summers and D. variabilis restricted to regions with humid summers (Wilkinson 1967). Further complicating matters, hybridization has long been known to occur among several closely related Dermacentor spp., including D. andersoni and D. variabilis (Cooley 1938, Oliver et al. 1972, Goddard et al. 2020). Indeed, laboratory hybrids of D. andersoni, D. occidentalis, and D. variabilis are described although subsequent generations have not been demonstrated (Oliver et al. 1972). Determining the extent to which D. variabilis may be displacing D. andersoni in this region, if at all, will require direct surveys for questing ticks from the field and identification of ticks on larger domestic and wildlife hosts in the region. However, the present study and work in other tick systems underscore that, given ongoing shifts in distribution, geographic origin should not be used as a deciding factor in tick identification, particularly when morphologically similar species are likely present (Mertins et al. 2010, Nieto et al. 2018, Xu et al. 2019, Goddard et al. 2020).
As has been reported by others, very few immature stages of Dermacentor spp. were submitted from dogs and cats in the present study; these stages preferentially feed on rodents and other small mammals in nature and are rarely found on domestic animals (Bishop and Trembley 1945, Dryden and Payne 2004). It is unlikely that these smaller stages were simply overlooked, as larvae or nymphs of other ixodid tick species, including Amblyomma americanum, Ixodes scapularis, and Rhipicephalus sanguineus, are routinely collected and submitted from pets (Little et al. 2018, Saleh et al. 2019). However, all D. andersoni recovered in the present study, and almost all D. variabilis (99.7%), were adults. In contrast, nymphs of the one-host tick D. albipictus are occasionally reported from pets in both the United States and Canada (Duncan et al. 2020). Adults—both male and female—of D. variabilis and D. andersoni are known to feed on a wide array of medium and large domestic and wild mammals, including horses, cattle, sheep, dogs, and cats, as well as deer and other wild cervids, coyotes, and bears, questing almost entirely in the warmer months throughout their range (Cooley 1938, Harwood and James 1979, Burg 2001, Kollars et al. 2000, Dryden and Payne 2004, Eisen et al. 2007). In the present study, collection of D. variabilis from dogs and cats similarly peaked in the summer months (Fig. 2).
While Dermacentor spp. ticks feeding on pets can cause direct harm, including blood loss and tick paralysis, members of this genus are also important vectors of pathogens capable of causing severe disease, including R. rickettsii and other spotted fever group Rickettsia spp. (SFGR; Burgdorfer 1975, McDade and Newhouse 1986, Dryden and Payne 2004, Parola et al. 2013). The expansion of D. variabilis populations suggested by other recent publications and supported by the findings in the present study further underscores the need to better understand the role of this tick as a vector of disease agents (Minigan et al. 2018, Lehane et al. 2020). Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever, is infrequently detected in D. variabilis, with surveys identifying the pathogen in 0.1% or fewer of ticks tested, if at all (Hecht et al. 2019, Luedtke et al. 2020, Occi et al. 2020). Recent work suggests that R. rickettsii also may be transmitted by ticks of other genera, including R. sanguineus sensu lato, A. americanum, and Haemaphysalis longicornis (Labruna et al. 2008, Levin et al. 2017, Stanley et al. 2020). Other SFGR, including R. montanensis and R. bellii, are more often identified in D. variabilis although they are widely suspected to have milder or unknown pathogenicity compared to R. rickettsii (Hecht et al. 2019, Sanchez-Vicente et al. 2019, Trout Fryxell et al. 2015, McQuiston et al. 2012, Stromdahl et al. 2011, Macaluso et al. 2002). Further research is warranted to better understand the veterinary and public health implications and the pathogenic potential of D. variabilis as a commonly encountered tick that appears to be more geographically widespread than is current recognized (Lehane et al. 2020). Together, our findings reinforce the need to accurately identify ticks both morphologically and molecularly and to continue to monitor distribution of D. variabilis as a critical aspect of tick-borne disease control (Lehane et al. 2020).
Acknowledgments
We are grateful to the veterinarians and technicians across the United States who took time out of their busy schedules to collect and submit ticks for this research. Funding for tick identification was provided by the Krull-Ewing Endowment, Oklahoma State University Foundation, National Institutes of Health (NIH) grant R03 AI149638-01, and National Science Foundation (NSF) grant (1920946). K.T.D. is the Boehringer Ingelheim Resident in Veterinary Parasitology through the National Center for Veterinary Parasitology. We also thank all members of the Krull-Ewing laboratory at Oklahoma State University for their support.
References Cited
- Bishop, F. C., and Trembley H. L.. . 1945. Distribution and hosts of certain North American ticks. J. Parasitol. 31: 1–54. [Google Scholar]
- Blagburn, B. L., Lindsay D. S., Vaughn J. L., Ripley N. S., Wright J. C., Lynn R. C., Kelch W. J., Ritchie G. C., Hepler D.. . 1996. Prevalence of canine parasites based on fecal flotation. Comp Cont Ed Pract Vet. 18: 483–509. [Google Scholar]
- Burg, J. G. 2001. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med. Vet. Entomol. 15: 413–421. [DOI] [PubMed] [Google Scholar]
- Burgdorfer, W. 1975. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol. 12: 269–278. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Geographic distribution of ticks that bite humans. Available from https://www.cdc.gov/ticks/geographic_distribution.html.
- Cooley, R. A. 1938. The genera Dermacentor and Otocentor (Ixodidae) in the United States, with studies in variation. U.S. National Institutes of Health Bulletin No. 171, 1–89. https://catalog.hathitrust.org/Record/100617333. [Google Scholar]
- Dergousoff, S. J., and Chilton N. B.. . 2007. Differentiation of three species of ixodid tick, Dermacentor andersoni, D. variabilis and D. albipictus, by PCR-based approaches using markers in ribosomal DNA. Mol. Cell. Probes 21: 343–348. [DOI] [PubMed] [Google Scholar]
- Dergousoff, S. J., Galloway T. D., Lindsay L. R., Curry P. S., and Chilton N. B.. . 2013. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 50: 510–520. [DOI] [PubMed] [Google Scholar]
- Duncan, K. T., Clow K. M., Sundstrom K. D., Saleh M. N., Reichard M. V., and Little S. E.. . 2020. Recent reports of winter tick, Dermacentor albipictus, from dogs and cats in North America. Vet. Parasitol. Reg. Stud. Reports. 22: 100490. [DOI] [PubMed] [Google Scholar]
- Dryden, M. W., and Payne P. A.. . 2004. Biology and control of ticks infesting dogs and cats in North America. Vet. Ther. 5: 139–154. [PubMed] [Google Scholar]
- Eisen, L., Meyer A. M., and Eisen R. J.. . 2007. Climate-based model predicting acarological risk of encountering the human-biting adult life stage of Dermacentor andersoni (Acari: Ixodidae) in a key habitat type in Colorado. J. Med. Entomol. 44: 694–704. [PubMed] [Google Scholar]
- Gibbons, R. J. 1939. Survey of Rocky Mountain spotted fever and sylvatic plague in western Canada during 1938. Can J Publ Health. 30: 184–187. [Google Scholar]
- Goddard, J., Allerdice M., Portugal J. S., Moraru G. M., Paddock C. D., and King J.. . 2020. What is going on with the genus Dermacentor? Hybridizations, introgressions, Oh My! J. Med. Entomol. 57: 653–656. [DOI] [PubMed] [Google Scholar]
- Guzmán-Cornejo, C., Robbins R. G., Guglielmone A. A., Montiel-Parra G., Rivas G., and Perez T. M.. . 2016. The Dermacentor (Acari, Ixodida, Ixodidae) of Mexico: hosts, geographical distribution and new records. Zookeys. 569: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood, R. F., and James M. T.. . 1979. Ticks and tick-associated diseases. Entomology in human and animal health, 7th ed, pp. 371–416. Macmillian Publishing, New York, NY. [Google Scholar]
- Hecht, J. A., Allerdice M. E. J., Dykstra E. A., Mastel L., Eisen R. J., Johnson T. L., Gaff H. D., Varela-Stokes A. S., Goddard J., Pagac B. B., . et al. 2019. Multistate Survey of American Dog Ticks (Dermacentor variabilis) for Rickettsia Species. Vector Borne Zoonotic Dis. 19: 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, A. M., Burdett C., McCool M. J., Fox A., and Riggs P.. . 2015. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the U.S.A. Med. Vet. Entomol. 29: 178–188. [DOI] [PubMed] [Google Scholar]
- Jones, E. O., Gruntmeir J. M., Hamer S. A., and Little S. E.. . 2017. Temperate and tropical lineages of brown dog ticks in North America. Vet. Parasitol. Reg. Stud. Reports. 7: 58–61. [DOI] [PubMed] [Google Scholar]
- Jongejan, F., and Uilenberg G.. . 2004. The global importance of ticks. Parasitology. 129(Suppl): S3–14. [DOI] [PubMed] [Google Scholar]
- Kollars, T. M., Jr, J. H.Oliver, Jr, Masters E. J., Kollars P. G., and Durden L. A.. . 2000. Host utilization and seasonal occurrence of Dermacentor species (Acari:Ixodidae) in Missouri, USA. Exp. Appl. Acarol 24: 631–643. [DOI] [PubMed] [Google Scholar]
- Labruna, M. B., Ogrzewalska M., Martins T. F., Pinter A., and Horta M. C.. . 2008. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J. Med. Entomol. 45: 1156–1159. [DOI] [PubMed] [Google Scholar]
- Lehane, A., Parise C., Evans C., Beati L., Nicholson W. L., and Eisen R. J.. . 2020. Reported county-level distribution of the American dog tick (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol. 57: 131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. L., Zemtsova G. E., Killmaster L. F., Snellgrove A., and Schumacher L. B. M.. . 2017. Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick. Borne. Dis. 8: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, E. E., Galloway T. D., Artsob H., Lindsay L. R., Drebot M., Wood H., and Robbins R. G.. . 2016. A handbook to the ticks of Canada (Ixodida: Ixodidae, Argasidae). Biological Survey of Canada, Monograph No. 7, Ottawa, 317p. [Google Scholar]
- Little, S. E. 2013. Future challenges for parasitology: vector control and one health in the Americas. Vet. Parasitol. 195: 249–255. [DOI] [PubMed] [Google Scholar]
- Little, S. E., Barrett A. W., Nagamori Y., Herrin B. H., Normile D., Heaney K., and Armstrong R.. . 2018. Ticks from cats in the United States: patterns of infestation and infection with pathogens. Vet. Parasitol. 257: 15–20. [DOI] [PubMed] [Google Scholar]
- Luedtke, B. E., Shaffer J. J., Monrroy E., Willicott C. W., and Bourret T. J.. . 2020. Molecular detection of spotted fever group Rickettsiae (Rickettsiales: Rickettsiaceae) in Dermacentor variabilis (Acari: Ixodidae) collected along the Platte River in South Central Nebraska. J. Med. Entomol. 57: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso, K. R., Sonenshine D. E., Ceraul S. M., and Azad A. F.. . 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39: 809–813. [DOI] [PubMed] [Google Scholar]
- MacDonald, A. J. 2018. Abiotic and habitat drivers of tick vector abundance, diversity, phenology and human encounter risk in southern California. PLoS One 13: e0201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade, J. E., and Newhouse V. F.. . 1986. Natural history of Rickettsia rickettsii. Annu. Rev. Microbiol. 40: 287–309. [DOI] [PubMed] [Google Scholar]
- McQuiston, J. H., Zemtsova G., Perniciaro J., Hutson M., Singleton J., Nicholson W. L., and Levin M. L.. . 2012. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 12: 1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins, J. W., Moorhouse A. S., Alfred J. T., and Hutcheson H. J.. . 2010. Amblyomma triste (Acari: Ixodidae): new North American collection records, including the first from the United States. J. Med. Entomol. 47: 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigan, J. N., Hager H. A., Peregrine A. S., and Newman J. A.. . 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick. Borne. Dis. 9: 354–362. [DOI] [PubMed] [Google Scholar]
- Nadolny, R. M., Wright C. L., Hynes W. L., Sonenshine D. E., and Gaff H. D.. . 2011. Ixodes affinis (Acari: Ixodidae) in southeastern Virginia and implications for the spread of Borrelia burgdorferi, the agent of Lyme disease. J. Vector Ecol. 36: 464–467. [DOI] [PubMed] [Google Scholar]
- Nieto, N. C., Porter W. T., Wachara J. C., Lowrey T. J., Martin L., Motyka P. J., and Salkeld D. J.. . 2018. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS One 13: e0199644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occi, J., Egizi A. M., Goncalves A., and Fonseca D. M.. . 2020. New Jersey-wide survey of spotted fever group Rickettsia (Proteobacteria: Rickettsiaceae) in Dermacentor variabilis and Amblyomma americanum (Acari: Ixodida: Ixodidae). Am. J. Trop. Med. Hyg. 103: 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. H., Jr, Wilkinson P. R., and Kohls G. M.. . 1972. Observations on hybridization of three species of North American Dermacentor ticks. J. Parasitol. 58: 380–384. [PubMed] [Google Scholar]
- Parola, P., Paddock C. D., Socolovschi C., Labruna M. B., Mediannikov O., Kernif T., Abdad M. Y., Stenos J., Bitam I., Fournier P. E., . et al. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev. 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh, M. N., Sundstrom K. D., Duncan K. T., Ientile M. M., Jordy J., Ghosh P., and Little S. E.. . 2019. Show us your ticks: a survey of ticks infesting dogs and cats across the United States. Parasit. Vectors. 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vicente, S., Tagliafierro T., Coleman J. L., Benach J. L., and Tokarz R.. . 2019. Polymicrobial nature of tick-borne diseases. mBio. 10: e02055– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine, D. E. 2018. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, H. M., Ford S. L., Snellgrove A. N., Hartzer K., Smith E. B., Krapiunaya I., and Levin M. L.. . 2020. The ability of the invasive Asian longhorned tick Haemaphysalis longicornis (Acari: Ixodidae) to acquire and transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the agent of rocky mountain spotted fever, under laboratory conditions. J. Med. Entomol. 57: 1635–1639. [DOI] [PubMed] [Google Scholar]
- Stromdahl, E. Y., Jiang J., Vince M., and Richards A. L.. . 2011. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis. 11: 969–977. [DOI] [PubMed] [Google Scholar]
- Thomas, J. E., Staubus L., Goolsby J. L., and Reichard M. V.. . 2016. Ectoparasites of free-roaming domestic cats in the central United States. Vet. Parasitol. 228: 17–22. [DOI] [PubMed] [Google Scholar]
- Trout Fryxell, R. T., Steelman C. D., Szalanski A. L., Billingsley P. M., and Williamson P. C.. . 2015. Molecular detection of Rickettsia species within ticks (Acari: Ixodidae) collected from Arkansas United States. J. Med. Entomol. 52: 500–508. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture . 1976. Ticks of veterinary importance. In: Agriculture Handbook No. 485. https://naldc.nal.usda.gov/download/CAT87208761/PDF. [Google Scholar]
- Wilkinson, P. R. 1967. The distribution of Dermacentor ticks in Canada in relation to bioclimatic zones. Can. J. Zool. 45: 517–537. [DOI] [PubMed] [Google Scholar]
- Wood, H., Dillon L., Patel S. N., and Ralevski F.. . 2016. Prevalence of Rickettsia species in Dermacentor variabilis ticks from Ontario, Canada. Ticks Tick. Borne. Dis. 7: 1044–1046. [DOI] [PubMed] [Google Scholar]
- Xu, G., Pearson P., Dykstra E., Andrews E. S., and Rich S. M.. . 2019. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 19: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunik, M. E., Galloway T. D., and Lindsay L. R.. . 2015. Ability of unfed Dermacentor variabilis (Acari: Ixodidae) to survive a second winter as adults in Manitoba, Canada, near the Northern Limit of their range. J. Med. Entomol. 52: 138–142. [DOI] [PubMed] [Google Scholar]