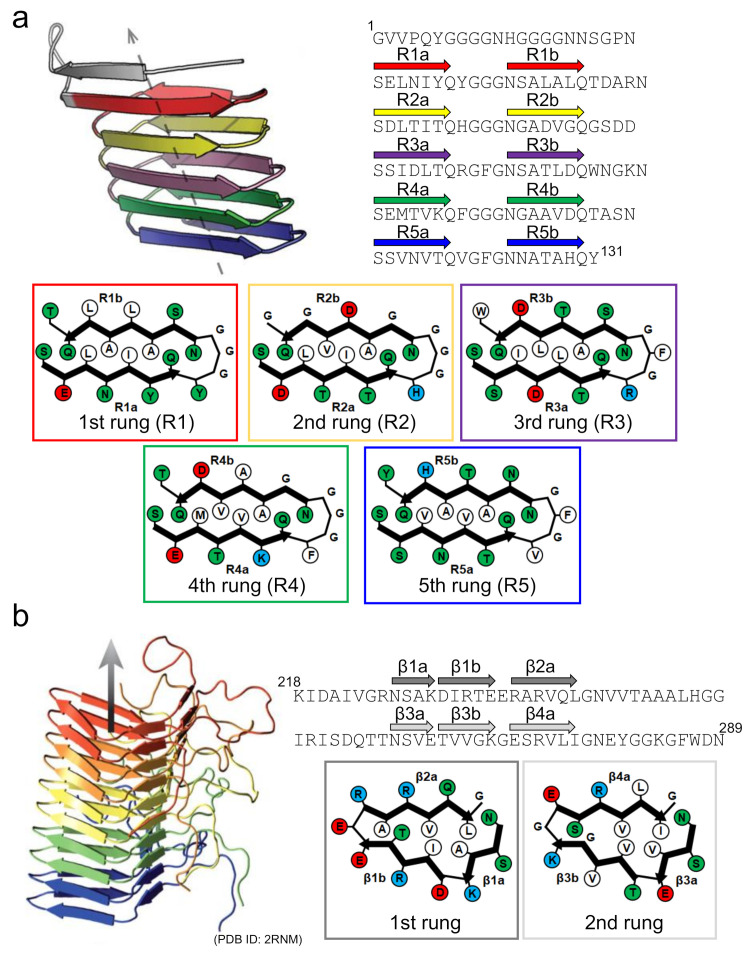
Figure 6.
Structures of functional amyloids consisting of a β-solenoid structure. (a) The side view of the protofilament and the schematic arrangement of side chains within the protofilament of CsgA. The left-handed structure, which is one of the low-energy conformations predicted by the computer simulation, is shown as a representative example. The side view was reproduced from [64], with permission. Copyright (2014) American Chemical Society. The schematic arrangement of side chains was drawn with reference to [64]. A single polypeptide chain takes a 5-rung solenoid structure, and each rung is shown in a different color. In the schematic illustrations of the arrangements of side chains at each rung, hydrophobic, polar, positively charged, and negatively charged residues are colored in white, green, blue, and red, respectively. The R1b strand is possibly unstable compared to the other ones according to the simulated structure. (b) Side view of the protofilament and a schematic illustration of the side chain arrangements within the protofilament of HET-s(218-289). Reproduced with modification from [67], with permission. Copyright (2008) Springer Nature. In the side view, five polypeptide chains constituting a β-solenoid structure, each of which takes a 2-rung solenoid, are shown in different colors. In the schematic illustrations of the arrangements of the side chains at each rung, hydrophobic, polar, positively charged, and negatively charged residues are colored in white, green, blue, and red, respectively.