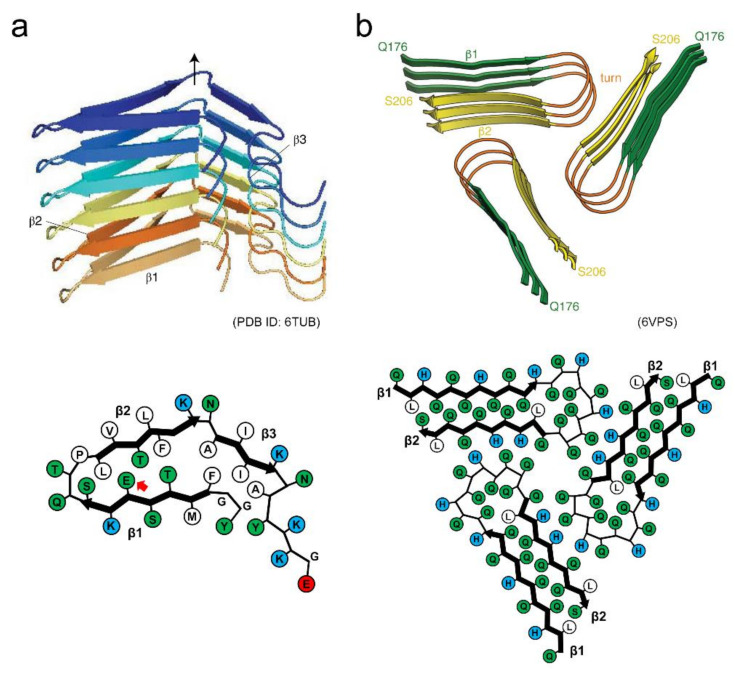
Figure 7.
Structures of functional amyloids showing high reversibility. (a) Side view of the protofilament and schematic illustration of the arrangement of side chains within the protofilament of β-endorphin. Reproduced with modification from [27], with permission. Copyright (2020) Springer Nature Limited. Each polypeptide chain is shown in a different color. In the schematic arrangement of the side chains, hydrophobic, polar, and positively charged residues are colored in white, green, and blue, respectively. The red arrow indicates the glutamate residue with the higher pKa value. (b) Side view of the filament and schematic illustration of the arrangement of side chains within the filament of Orb2. While each polypeptide chain is not distinguished by color, each strand within the polypeptide chain is shown in a different color instead. In the schematic arrangement of side chains, hydrophobic, polar, and positively charged residues are colored in white, green, and blue, respectively. Reproduced with modification from [28], with permission. Copyright (2020) American Association for the Advancement of Science.