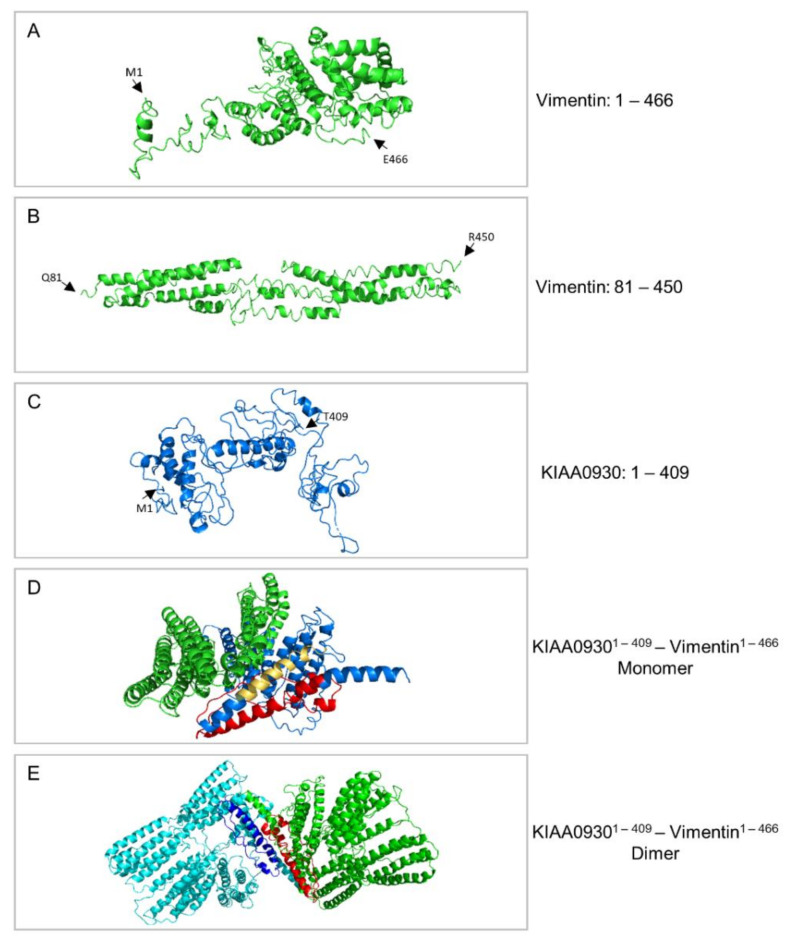
Figure 6.
Structural models of vimentin, KIAA0930, and KIAA0930-vimentin hybrid proteins. (A) Structural model of the human vimentin (VIM-201; ENST00000224237.9; amino acid residues: 1–466). (B) Model of a human vimentin in silico “truncated” version (amino acid residues: 81–450). Residues 1–80 and 451–466 that correspond to the amino- (“N”) and carboxyl- (“C”) terminus of the full-length vimentin, respectively, were not used, since they proved to obtain disordered conformations (A). (C) Structural model of the human KIAA0930 protein (KIAA0930-201; ENST00000251993.11; amino acid residues: 1–409). (D) Structural model of the KIAA0930-vimentin hybrid, monomer, protein (amino acid residues: 1–409(KIAA0930)/1–466(Vimentin)). The domain that corresponds to the KIAA0930 protein is colored in blue. Residues 22–99 (colored in red) and 111–132 (colored in orange/yellow) contain motifs that share characteristics with leucine zippers (LZs). (E) Theoretical model of a KIAA0930-vimentin hybrid protein homodimer derived from the docking experiments. In each monomer, residues 22–99 are colored in red and blue, respectively, and are located at the interface of interacting monomers. “M”: Methionine. “E”: Glutamic Acid. “Q”: Glutamine. “R”: Arginine. “T”: Threonine.