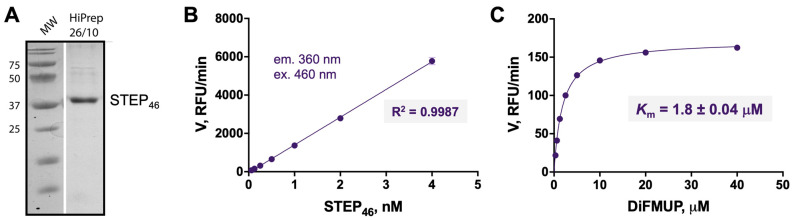
Figure 3.
(A) SDS PAGE of full-length human STEP46 (MW 44.3 kDa) after expression of HIS-tagged fusion protein using a custom-made codon-optimized vector, and purification using Ni-affinity chromatography and subsequent processing over an S75 size exclusion chromatography column. (B) Titration of STEP46 in a phosphatase activity assay in 384-well format using 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP, 50 μM) as a substrate. Fluorescence intensity was measured in kinetic mode to determine the initial rates (V) from the slopes of the progress curves. The initial rates were found highly correlative with the various STEP46 concentrations tested, as demonstrated by the linear regression coefficient (R2). (C) Michaelis–Menten kinetics for STEP46 (0.5 nM) and DiFMUP using a similar assay format as described in (B). The Michaelis–Menten constant (Km) was calculated by fitting initial rates to the Michaelis–Menten equation using the program GraphPad Prism.