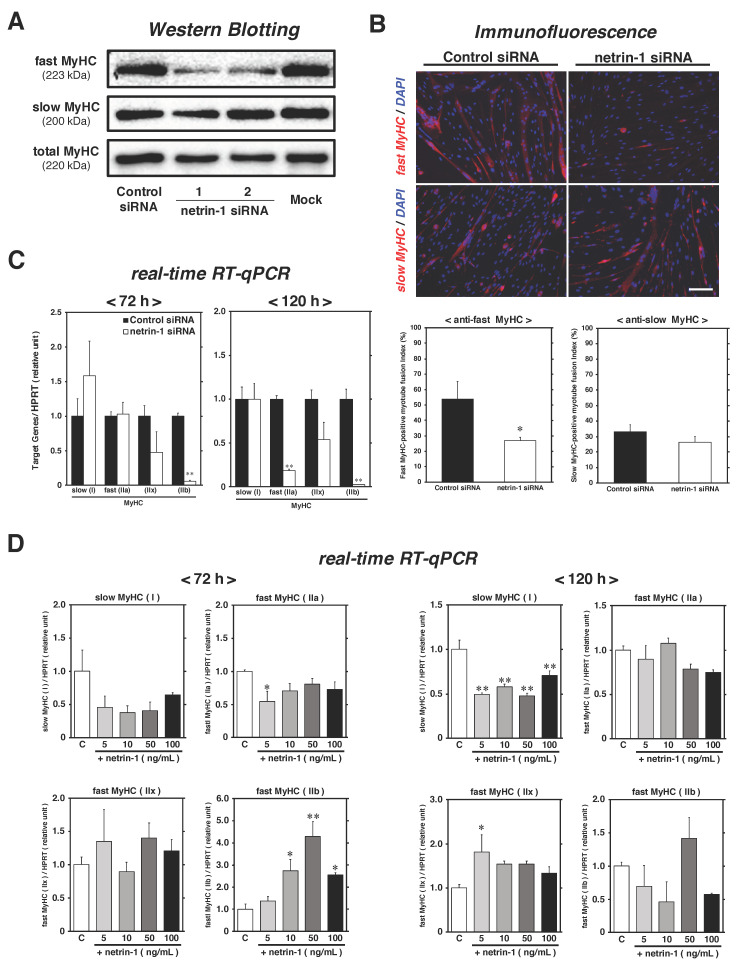
Figure 5.
Significant reduction in the expression of fast-MyHC was observed following netrin-1 expression knockdown, and in the slow-MyHC expression level was found following netrin-1 addition to satellite cells. As described in Figure 4, proliferating satellite cell-derived myoblasts were maintained in DM with netrin-1 siRNA until 72 h or 120 h. (panel A) The protein expression of fast MyHC (contained type IIa, IIx, and IIb) and slow MyHC (type I) was detected with Western Blotting using cell lysates after normalization to the expression level of total MyHC. (panel B) Immunofluorescence microscopy for fast/slow MyHC (red) and DAPI (blue) shows fast-/slow-twitch myotubes and nuclei. Scale bar in panel, 100 μm. Myotube fusion index of each myofiber type was evaluated and defined as the ratio of myonuclei on myotubes containing more than two nuclei. The results are expressed as the mean ± SEM of three independent cultures. (panel C) The cell lysates were analyzed for the mRNA expression of MyHC type I, IIa, IIx, and IIb by real-time RT-qPCR standardized to HPRT. The bars depict the mean ± SEM of three independent cultures by relative units, as compared to control siRNA-treated cultures. Significant differences from control siRNA cultures (black bar) at p < 0.05 or p < 0.01 are indicated by single or double asterisks. (panel D) As well as in Figure 4E, satellite cell primary cultures in DM were respectively added recombinant netrin-1 (0, 5, 10, 50, and 100 ng/mL) at 48 h post-differentiation and maintained until 72 h or 120 h. The mRNA expression levels of MyHC type I, IIa, IIx, and IIb isoforms were evaluated by real-time RT-qPCR and were normalized to the level of HPRT at all cultures indicated. The bars depict the mean ± SEM of three independent cultures by relative units, as compared to control culture. Significant differences from the control culture (the left-most open bar) at p < 0.05 and p < 0.01 are indicated by single and double asterisks, respectively. C: control culture.