Abstract
COVID-19 is a kind of SARS-CoV-2 viral infectious pneumonia. This research aims to perform a bibliometric analysis of the published studies of vitamins and trace elements in the Scopus database with a special focus on COVID-19 disease. To achieve the goal of the study, network and density visualizations were used to introduce an overall picture of the published literature. Following the bibliometric analysis, we discuss the potential benefits of vitamins and trace elements on immune system function and COVID-19, supporting the discussion with evidence from published clinical studies. The previous studies show that D and A vitamins demonstrated a higher potential benefit, while Selenium, Copper, and Zinc were found to have favorable effects on immune modulation in viral respiratory infections among trace elements. The principles of nutrition from the findings of this research could be useful in preventing and treating COVID-19.
Keywords: COVID-19, Vitamins, Trace elements, Immune system, Bibliometric analysis
1. Introduction
As a positive-sense single-stranded RNA genome, coronaviruses are found in a variety of animals and humans [1,2]. It is a widespread family of viruses that provoke respiratory diseases. Coronavirus respiratory symptoms range from the common cold to severe lung infections [3]. SARS-CoV-2, found in China in December 2019, is genetically similar to SARS-CoV. SARS-CoV virus caused an acute respiratory distress syndrome (ARDS) outbreak in 2002 [4,5]. This novel coronavirus is however unfamiliar to the human immune system. Thus, no natural immune system has therefore been established for this virus. Perhaps this is why SARS-CoV-2 spreads so quickly worldwide.
The immune system has special roles in the defense against infection [6]. In fact, it is recognized as the body's defense against infections [7] which involves members that participate in adaptive immune and innate immune systems. Importantly, both groups work together to defend themselves strongly against invaders [8]. The more primitive innate immune system includes exterior defenses, different phagocytic cells, cytokine groups, interferons, and serum proteins that offer a frontline of viral protection against infection [9,10]. Most viral infections are seen to be controlled by the innate immune system. However, the adaptive response must be mobilized if viral replication outpaced innate defenses. Adaptive immunity is mediated by two major components, B and T cells. The antigen-specific memory cells characterize these cells. The virus can be neutralized through host T-lymphocyte cells [[11], [12], [13]]. Chen et al. [10] address the role of the immune system and vital molecules in "antiviral defense" against influenza A viruses. It was found that if there is significant cell proliferation in the immune response, the increasing of the number of defensive immune cells needs some main components such as RNA, DNA, protein and complex lipid synthesis.
Many different minerals and vitamins are essential as cofactors in the metabolic machinery involved in biosynthesis and energy generation. For example, the human immunity of pediatric patients following influenza vaccination was improved with vitamin A and D supplementation [14]. The immune improvement in patients with Torque Teno virus (TTV) has been shown by high-dose zinc supplementation [15]. Likewise, the supplementation of selenium has shown a positive response to a challenge of the influenza vaccine [16]. Therefore, maximizing natural immunity is extremely important for the prevention and minimization of the symptoms of a human body virus [17]. To date, there is no robust and particular therapy against the novel coronavirus. While it is not well understood what the pathogenesis of the novel coronavirus is, similar mechanisms of other infectious diseases (e.g., SARS-CoV, influenza, and MERS-CoV) can still provide valuable information regarding the SARS-CoV-2 pathogenesis to help us recognize, prevent and protect COVID-19 outbreak. There are several trace elements as well as vitamins which are necessary to support the immune system’s normal functioning [7,18] which revealed a significant impact on improving immunity against viral infections. The intake of vitamin-rich foods can also help to boost the virus immune system [19]. According to the literature, it is found that the use of vitamins may be beneficial in the treatment of coronavirus [17]. Accordingly, this review aimed to investigates the role of trace elements and vitamins to boost immune system function to fight against the COVID-19 pandemic. Hence, based on the above discussion, we present the following hypothesis:
-
•
Vitamins and trace elements have a beneficial role in immune system improvement and may be effective in reducing the risk of SARS-CoV-2 infection.
This study is structured into the following sections. In Section 2, we present the bibliometric analysis. In Section 3, we present the role of vitamins in immune system function. In Section 4, we present the role of trace elements in immune system function. Finally, we conclude and summarize the outcomes in Section 5. In Section 6, we present the recommendations and future work. In Table 1 , the list of acronyms used in this study is presented.
Table 1.
List of Acronyms Used in This Study.
| Abbreviation | Term |
|---|---|
| ARDS | Acute Respiratory Distress Syndrome |
| B2 Vitamin | Riboflavin |
| B3 Vitamin | Niacin |
| B6 Vitamin | Pyridoxine |
| B12 Vitamin | Cobalamin |
| B Cells | Bone Marrow Cells |
| BVDV | Bovine Viral Diarrhea Virus |
| CD8 | Cluster of Differentiation 8 (Glycoprotein) |
| CHB | Chronic Hepatitis Virus B |
| COVID-19 | Coronavirus Disease of 2019 |
| D3 Vitamin | Cholecalciferol |
| DNA | Deoxyribonucleic Acid |
| g | Gram |
| H1N1 | Hemagglutinin Type 1 and Neuraminidase Type 1 |
| HCV | Hepatitis C Virus |
| HIV | Human Immunodeficiency Virus |
| IV Vitamin C | Intravenous Vitamin C |
| IVB | Influenza Virus B |
| IU | International Unit |
| LF | Links Feature |
| LRTI | Lower Respiratory Tract Infection |
| MERS-CoV | Middle East Respiratory Syndrome Coronavirus |
| MeV | Measles Virus |
| mg | Milligram |
| nCOV | Novel Coronavirus |
| RCT | Randomized Controlled Trial |
| RNA | Ribonucleic Acid |
| SARS-CoV | Severe Acute Respiratory Syndrome Coronavirus |
| T Cells | Thymus Cells |
| TGFβ | Transforming Growth Factor Beta |
| TLS | Total Link Strength |
| TTV | Torque Teno Virus |
| μg | Microgram |
| WOS | Web of Science |
| ZN2 | Intracellular Free Zinc |
2. Bibliometric analysis
2.1. Methodology
Bibliometrics analysis is the deployment of statistic-based approaches to inspect the published materials in a particular discipline [20]. Bibliometric measures based on published studies can be utilized to assess the research outcomes and the citation-based influence of the research [21].
In this study, the Scopus database was used to retrieve related researches, in which only journal articles were included in the analysis. To conduct the bibliometric analysis, we retrieved two groups of publications. To retrieve the Group-1 of papers we used the next keywords:("Vitamin" OR "Vitamins") AND ("COVID-19″ OR "Coronavirus" OR "COVID" OR "nCoV disease" OR "SARS-CoV-2″).To retrieve the Group-2 of papers we used the following keywords: ("Trace elements") AND ("COVID-19″ OR "Coronavirus" OR "COVID" OR "nCoV disease" OR "SARS-CoV-2″). After retrieving the two groups of publications, we performed multiple bibliometric analyses using VOSviewer software [22].
Bibliometric analysis was carried out using the VOSviewer tool. VOSviewer focuses on locating elements in two-dimensional diagrams [23]. Bibliographic data can be utilized to develop a web of citation, co-citation, co-authorship, co-occurrence, or bibliographic coupling links. Based on the kind of path, several kinds of elements are provided. The interval between two elements indicates the resemblance or relationship of the elements as precisely as possible [24]. The VOSviewer software has multiple functionalities to enable handling the diagrams appropriately: zoom, search, and scroll to allow a comprehensive investigation of the diagram. The program presents various visualizations, enabling the reader to concentrate either on the diagram’s general layout or on its more specific features [25]. In subsections 2.3, 2.4, and 2.6, we used several visualizations to represent the outcomes of the analyses: network mapping, item density mapping, and cluster density mapping.
In the network mapping, elements are presented by their titles and represented in the diagram by circles or frames. The size of the title and circle is indicated by the weight of the item it represents. The greater the weight of an element, the bigger the title and the frame of the element. Titles may not be presented for some elements to prevent overlapping titles. The element’s color indicates the segment of the element. Paths among elements represent connections between these elements. Maximum, 500 associations are presented, indicating the strongest associations among elements.
In the item density mapping, elements are depicted by comparably indicating their titles as in the network mapping and the overlay mapping [26]. Each element in the diagram is colored based on its density [27]. Colors vary from red to blue to green. The higher the number of elements in the area of an element and the greater the weights of the surrounding elements, the more closely the color of an element is to red. On the contrary, the less the number of elements in the surrounding area of an element and the less the weights of the surrounding elements, the more closely the color of the element is to blue [25].
The cluster density diagrams can be provided when elements belong to a particular segment. These diagrams resemble item density mapping, only that, the elements’ density is presented individually for each group of elements. Also, the color of an element in the diagram is resulted by combining the colors of various segments. The weight indicated to the color of a specific segment is resulted by the count of elements that are assigned to that segment in the surrounding area of a particular spot.
To conduct the bibliometric analysis, we performed a two-fold search process using the Scopus database. Based on the identified keywords, we retrieved two groups of publications, related to (1) vitamins and COVID-19 and (2) trace elements and COVID-19. We presented the protocol we followed in Fig. 1 .
Fig. 1.
Bibliometric Analysis Methodology.
In Group-1, we retrieved 833 initial studies. By including only journal articles and excluding other types of publications such as letters to the editor, book chapters, and conference studies, we obtained 354 studies. In Group-2, we initially retrieved 73 studies. Following the inclusion of the journal articles and excluding other types of studies, we kept only 30 studies. The bibliometric analyses entail several procedures that include: subject analysis, term analysis, keyword analysis, country analysis, and citation analysis, which we will clarify in the coming subsections.
2.2. Subject analysis
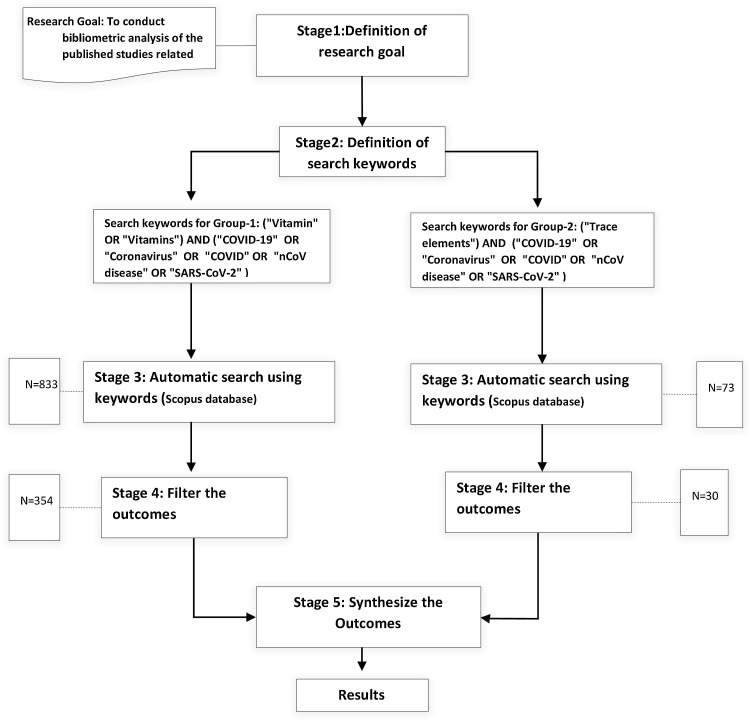
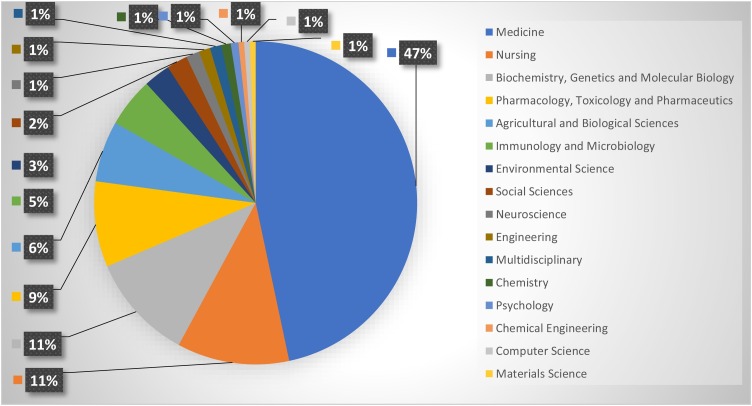
The research subject is the main field or area that each research belongs to. All publications should belong to one or more clear areas. Hence, referring to the data analysis, the published studies belong to various and several disciplines. Among these disciplines: “Medicine”, “Nursing”, and “Biochemistry, Genetics and Molecular Biology” are the most subjects that the explored studies belong to, in both groups of studies. In Group-1, as indicated in Fig. 2 , the published studies belong to 16 various disciplines, which indicates the heterogeneity of the research topic. On the other hand, as indicated in Fig. 3 , Group-2 studies belong to 9 various disciplines. This can be explained by the total number of the publications covered in the analysis in Group-2 studies (30 studies), compared to Group-1 studies (354 studies).
Fig. 2.
Distribution of Publications per Subject of “Vitamins” and “COVID-19” Publications.
Fig. 3.
Distribution of Publications per Subject of “Trace Elements” and “COVID-19” Publications.
2.3. Term analysis
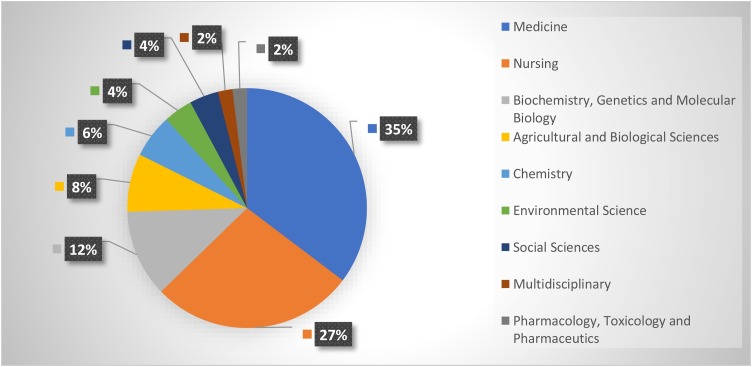
An investigation of the terms that occurred in the abstracts and titles of the retrieved studies can present perceptions about the basic themes and study directions about the role that vitamins and trace elements can play during the COVID-19 crisis. VOSviewer was utilized to inspect and envision the terms. Bibliographic details can be presented to VOSviewer in WOS, Scopus, Crossref JSON, PubMed, or RIS databases. These records can be utilized to provide VOSviewer with text details. Text details are usually mined from the title and abstract elements. Text details should be utilized to build a web of co-occurrence paths among terms. Term analysis in this study was performed by creating a map based on the bibliographic database file obtained from the Scopus database. Items are defined in the included text by utilizing particular natural language processing approaches. The outcomes of the term analysis of the two groups of publications are displayed in Fig. 4 . and Fig. 5 . The circle’s size indicates the occurrences of a word. The general distance among words presents insights into their relatedness. The smaller the distance between two words, the higher their link. The relationships of words are specified by calculating how many times the words appear simultaneously in the titles and abstracts [28]. The colors are used to distinguish among different clusters.
Fig. 4.
Terms Analysis of “Vitamins” and “COVID-19” Publications: a) Network Visualization and b) Density Visualization.
Fig. 5.
Terms Analysis of “Trace elements” and “COVID-19” Publications: a) Network Visualization and b) Density Visualization.
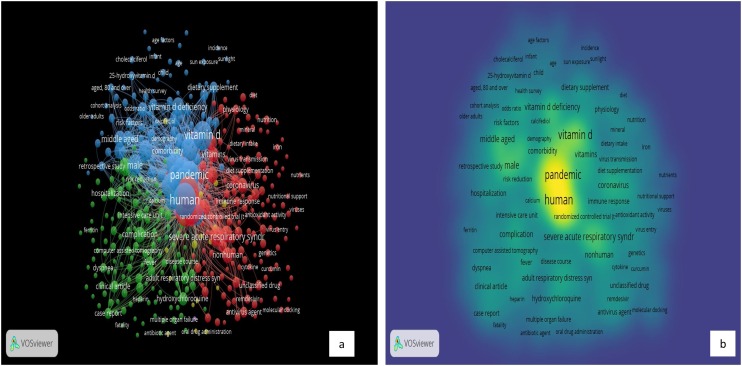
In Fig. 4 the term visualization presents how the words of the research topic are segmented together, and indicates four separate segments: Segment 1: red color, Segment2: green color, Segment 3: blue color, and Segment 4: yellow color. The total included items in the 4 clusters are 162 terms, 6606 links, and total link strength of 48854. The red segment includes 57 items, the green segment includes 41 items, the blue segment includes 36 items, and the yellow segment entails 28 items. Fig. 4 indicates that each of the four segments has one or more core words around which other words are placed. The four central terms are “COV”, “Vitamin D Deficiency”, “Virus”, and “Group”. The size of the font is utilized to exhibit the number of publications in which the word occurs.
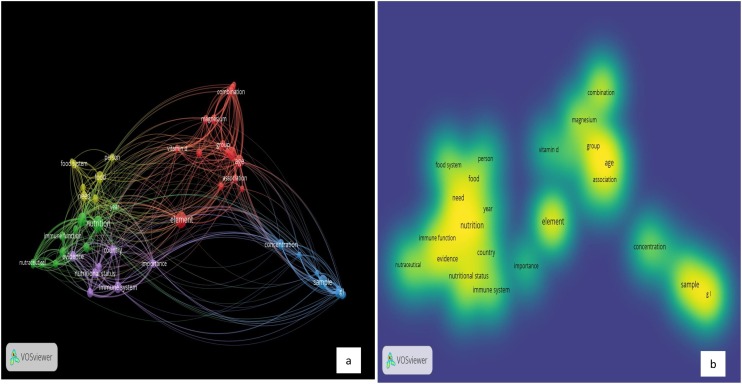
In Fig. 5 the term visualization presents how the words in Group-2 studies are segmented together, and indicates five separate segments: Segment 1: red color, Segment2: green color, Segment 3: blue color, Segment 4: yellow color, and Segment 5: purple color. The total included items in the 5 clusters are 49 terms, 442 links, and total link strength of 2828. The red segment includes 15 items, the green segment includes 11 items, the blue segment includes 9 items, the yellow segment includes 7 items, and the purple segment entails 7 items. The five central terms in the five segments are “Element”, “Nutrition”, “Sample”, “Food”, and “Immune System”, in the red, green, blue, yellow, and purple segments, respectively.
2.4. Keywords analysis
Focusing on keywords, the occurrences feature refers to how many publications in which a keyword exists. We performed a co-occurrence analysis based on keywords for Group-1 and Group-2 of publications. Co-occurrence analysis is based on the relationship between any two elements in the diagrams, which is indicated by the count of publications in which they presented together. Based on the analysis outcomes, in Group-1, the total included items in the 5 clusters are 379 terms, 32,520 links, and total link strength of 101372. The red segment includes 145 keywords, the green segment includes 124 keywords, the blue segment includes 91 keywords, the yellow cluster has 16 keywords, and the purple cluster has 3 keywords. The central term of cluster 1 is “Coronavirus Disease 2019” with 197 occurrences. This is expected as the main topic of the retrieved studies is based on COVID-19 disease. The central term of the green cluster is “adult” with 65 occurrences. The central terms of the blue cluster are “human” with 414 occurrences, “pandemic” with 169 occurrences, and “Vitamin D” with 165 occurrences. In this cluster, several terms related to Vitamin D were presented, with several forms; Vitamin D, Vitamin D deficiency, sunlight, and sun exposure. This indicates the correlation between COVID-19 and Vitamin D in the published studies. Fig. 6 presents the analysis of the keywords of “Vitamins” and “COVID-19” publications.
Fig. 6.
Keywords Analysis of “Vitamins” and “COVID-19” Publications: a) Network Visualization and b) Density Visualization.
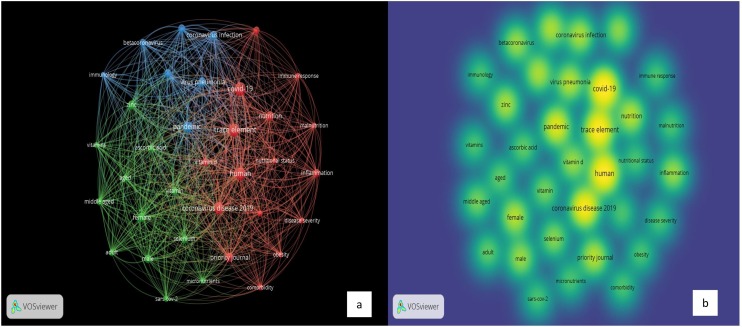
Based on the analyses outcomes, in Group-2, three clusters resulted, the total included items in the 3 clusters are 35 terms, 573 links, and total link strength of 2193. The red segment includes 16 keywords, the green segment includes 12 keywords, and the blue segment includes 7 keywords. Several keywords in the diagram were presented in each cluster. In the red cluster, the most dominant keywords are “human” with 22 occurrences, “coronavirus disease 2019” with 19 occurrences, “COVID-19” with 23 occurrences, and “Trace elements” with 21 occurrences. In the green cluster, the most dominant keywords are “female” with 11 occurrences, “male” with 9 occurrences, “selenium” with 8 occurrences, and “zinc” with 10 occurrences. In the blue cluster, the most dominant keywords are “pandemics” with 15 occurrences, and “virus pneumonia” with 11 occurrences. Fig. 7 presents keywords analysis of “Trace Elements” and “COVID-19” publications.
Fig. 7.
Keywords Analysis of “Trace Elements” and “COVID-19” Publications: a) Network Visualization and b) Density Visualization.
2.5. Country analysis
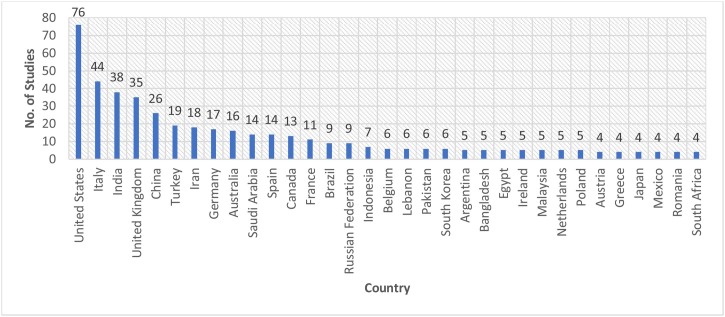
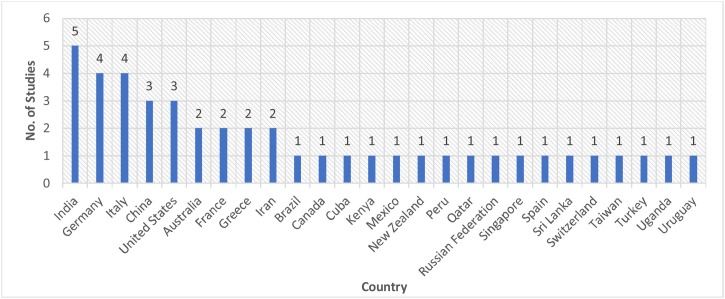
As represented in Fig. 8 , the United States was predominantly the most leading country for Group-1 publications with around 76 studies, followed by Italy with 44 studies, India with 38 publications, and United Kingdom with 35 studies. Meanwhile, Group 2 was dominated by publications from India with 5 studies, followed by Germany and Italy with 4 studies each, and China and the United States with 3 studies each, each of Australia, France, Greece, and Iran has 2 published studies. The rest of the countries listed in Fig. 9 has one published study each. These outcomes are based on the bibliographic database retrieved from the Scopus database. To present a clear figure, in Group-1 studies, we only include the countries that have more than three studies.
Fig. 8.
No of Studies per Country of Group-1 Studies.
Fig. 9.
No of Studies per Country of Group-2 Studies.
2.6. Citation per country
In this subsection, we present a citation visualization based on two-dimensional maps with countries as actors (Fig. 10, Fig. 11 ). Citation-countries visualization provides a clear display for the reader's eyes to perceive the structure of citations per country. A cluster density diagram is presented for countries that have been grouped by clusters. The density of countries is represented individually for each group of countries. The mapping of cluster density presents several colors in which each color resulted from a combination of colors of several countries. The color’s weight of a particular cluster is identified by the count of countries included in that segment in the surrounding area of the particular spot in the map.
Fig. 10.
Citation Visualization Map for Group-1 Studies.
Fig. 11.
Citation Visualization Map for Group-2 Studies.
Two weight features were utilized: (1) links feature (LF) and the (2) total link strength (TLS) feature. For a particular country, these features represent the number of associations of a country with other countries and the total strength of the associations of a country with other countries. For example, in the case of citation associations among countries, the LF represents the number of citation associations from a given country with other countries. The TLS feature represents the total strength of the citation associations of a particular country with other countries.
In the first group of publications, based on citations-country visualization, among 89 countries, 27 countries met the condition, as the least number of publications of a country is 5 and the least count of citations of a country is 0. The count of citations of a particular country is the total number of citations the publications of the country have indicated in the Scopus database. For each of the 27 countries, the total strength of the citation links with other countries was measured, and the countries with the highest TLS were selected. The highest group of linked elements entails 25 items, 8 clusters, 89 links, and 213 total link strength. Fig. 10 and Table 2 present the data of countries, citations, and total link strength for Group-1. Among the published articles in this group, the United Kingdom has received the highest number of citations (451), followed by Italy (371), then the United States (353), and China (332).
Table 2.
Countries with Highest Total Link Strength in Group-1 Studies.
| Country | Documents | Citations | Total Link Strength |
|---|---|---|---|
| United Kingdom | 35 | 451 | 82 |
| Italy | 44 | 371 | 54 |
| United States | 77 | 353 | 77 |
| China | 26 | 332 | 13 |
| India | 37 | 234 | 26 |
| Saudi Arabia | 14 | 142 | 5 |
| Ireland | 5 | 111 | 7 |
| Australia | 16 | 104 | 13 |
| Spain | 14 | 85 | 20 |
| Egypt | 5 | 83 | 2 |
| Germany | 17 | 77 | 13 |
| Iran | 18 | 70 | 13 |
| Belgium | 6 | 57 | 10 |
| Turkey | 19 | 51 | 6 |
| Canada | 13 | 43 | 22 |
| Brazil | 9 | 27 | 12 |
| France | 11 | 27 | 15 |
| Lebanon | 6 | 22 | 5 |
| Poland | 5 | 21 | 10 |
| Malaysia | 5 | 17 | 2 |
| South Korea | 6 | 13 | 1 |
| Netherlands | 5 | 12 | 2 |
| Russian Federation | 9 | 8 | 8 |
| Bangladesh | 5 | 7 | 4 |
| Argentina | 5 | 2 | 0 |
| Indonesia | 7 | 0 | 0 |
| Pakistan | 6 | 0 | 4 |
In the second group of publications, based on citations-country, among 26 countries, only 9 met the condition, as the least count of publications of a country is 2 and least count of citations of a country is 0. For each of the 9 countries, the total strength of the citation associations with other countries was measured, and the countries with the greatest total link strength were selected. The resulted diagram entails 9 items, 9 clusters, and 0 links. Fig. 11 and Table 3 present the data of countries, citations, and TLS for Group-2. Among the published articles in this group, Australia and Greece have received the highest number of citations (77), followed by Italy (76), Germany (19), and the United States (10).
Table 3.
Countries with Highest Total Link Strength in Group-2 Studies.
| Country | Documents | Citations | Total Link Strength |
|---|---|---|---|
| Australia | 2 | 77 | 0 |
| China | 3 | 0 | 0 |
| France | 2 | 0 | 0 |
| Germany | 4 | 19 | 0 |
| Greece | 2 | 77 | 0 |
| India | 4 | 3 | 0 |
| Iran | 2 | 1 | 0 |
| Italy | 4 | 76 | 0 |
| United States | 3 | 10 | 0 |
3. Part of vitamins in immune system function
The adequate status and intake of all vitamins are important for the prevention of suppressed immunity. It is found that suppressed immunity can cause predisposes to aggravates undernutrition and infections [29]. Following the bibliometric analysis of the published studies, the potential effects of major vitamins and their impact on the immune system are covered and briefly discussed as follows.
3.1. Vitamin A
Vitamin A is often referred to as an "anti-infectious" vitamin and plays an important role in infection defenses from its sufficient supply [30]. For the immune system’s function [31] and the function of several immune cell types and acute phase response [32], this vitamin is extremely important. This vitamin also has an anti-inflammatory role in several inflammatory conditions such as broncho-pulmonary dysplasia [33,34]. Hence vitamin A deficiency involves increased risks for a range of infections and altered immune responses [7,35]. Vitamin A effects have been investigated clinically on humans or experimentally on mice on the immune system against infectious agents [36]. The supplementation of vitamin A has been reported to reduce morbidity and mortality in measles pneumonia, measles virus (MeV), diarrheal disease, HIV infection, and malaria [[37], [38], [39], [40], [41]]. The deficiency of vitamin A was found to be highly entailed in measles and diarrhea so that measles in vitamin A-deficient children can become severe [42]. In Trottier, Colombo [43], the mechanism for vitamin A and retinoids for the innate immune response of the uninfected bystander T cells are discussed. Furthermore, it was found that vitamins A and D have an enhanced immune response to the influenza virus in children (2–8 years) [44]. In addition, in the chickens fed a marginally insufficient diet with vitamin A, the effect of infectious bronchitis virus or IVB, which is a type of coronavirus, was greater than that of a diet adequate with vitamin A [45]. According to the above discussion on vitamin A, it could be concluded that as this vitamin can enhance the immune response against infectious diseases, it may be an appropriate option for the treatment and prevention of this new coronavirus. However, more clinical and consumers’ data analyses are needed to reach a robust conclusion in this regard.
3.2. Vitamin C
As an ascorbic acid and water-soluble vitamin [30], vitamin C plays a protective role because the functions of the immune system are supported by this vitamin. This vitamin is necessary to develop and repair all body [46]. The levels of this vitamin may be depleted during infection and the demand for this vitamin increases with infection severity [47]. In the initial phase of viral infection, this vitamin has shown to be a key factor in anti-viral immune response [48]. Studies also showed that RNA and DNA viruses can be deployed by vitamin C as an inactivating agent, thereby decreasing viral infectivity [49,50]. Ascorbic acid can also detoxify viral products that cause inflammation and pain [51]. High-dose IV Vitamin C against viral conditions such as cold rhinovirus, Chikungunya, Zika, H1N1, avian virus, and flu is effective [52,53]. Oral vitamin C supplements (above 3 g doses) are also beneficial for preventing and treating respiratory and systemic infections [54]. Vitamin C also promotes immune function and protects against SARS coronavirus infection [55]. Another publication indicated that vitamin C increases chick embryo tracheal organ resistance to avian coronavirus [56]. Research in the USA previously carried out in 167 sepsis-related ARDS patients showed that 4-days IV vitamin C administration of ∼ 15 g / day may reduce death in those patients [57]. The IV vitamin C trial of patients with pneumonia [58] has shown no significant benefits for systemic corticosteroid treatment in COVID-19 patients [59]. Cheng [60] showed that high-dose intravenous vitamin C has been successfully used to treat 50 patients in China with mild to severe COVID-19. A new clinical trial for the COVID-19 patients’ treatment with infected pneumonia recently started in China, to investigate the effects of IV vitamin C. The researchers of this study expect the trial to be completed by the end of September [47]. Overall, it is found that the novel coronavirus is reported to cause lower respiratory tract infection. Accordingly, it is found from previous reports that vitamin C can be one of the appropriate treatment options for this virus.
3.3. Vitamin D
As a fat-soluble vitamin, vitamin D is found to be beneficial in modulating both adaptive and innate immune responses [61]. The results of the study by Grant, Lahore [62] demonstrated that vitamin D can reduce the risk of infections by several mechanisms. In most immune cells, receptors of this vitamin have been identified and some immune system cells are able to synthesize the active form of this vitamin from its precursor. This indicates that vitamin D has important immunoregulatory properties. In addition, this vitamin can enhance epithelial integrity [63,64] and directly improve host defense. Through the 156 reviews in evaluating the possible mechanisms, the results showed that vitamin D can have an important modulatory role for innate immune responses which is important in the prevention of respiratory viral infections [65]. A clinical trial has also reported a reduction in the infection of the dengue virus with 4000 IU/d of vitamin D [66,67] and HIV-1 [68]. Similarly, the recent RCT for the impact of this vitamin supplementation demonstrated the fact that it is able to promote higher plasma TGFβ levels without any improvement in antibody production [69]. In addition, the incidence of acute respiratory inflammation in older long-term patients has decreased by vitamin D [70]. In addition, a prior review suggested the use of vitamin D to decrease the risks of COVID-19 infection [71]. Moreover, the effect of vitamin D3 to reduce the influenza infection risk and COVID-19 was investigated in [72]. Further, according to the report in [73], the decreased state of vitamin E and vitamin D in calves has caused bovine viral diarrhea or BVDV infection.
In addition, the supplementation of vitamin D also upregulates the expression of antioxidation genes [74]. The increased production of glutathione as an antioxidant reduces the use of ascorbic acid with antimicrobial action [75,76], which is found to be effective in COVID-19 prevention and treatment [71]. In Ilie, Stefanescu [77], the levels of vitamin D have been shown to have major crude linkages to the COVID-19 cases and, in particular, the mortality resulting from COVID-19 infection. Furthermore, McCartney and Byrne [78] recommended supplementation of 20−50 μg/d of vitamin D to enhance the resistance of the older adults, nurses, homeless persons, and other vulnerable groups (for example, smokers care providers, vegetarians, vegans, overweight, and obese) to COVID-19, which should be extended rapidly to the general adult population. Alipio [79] also revealed that a vitamin D supplement may improve the clinical outcomes of COVID-2019-infected patients by an increase in serum (OH)D level. Thus, proper vitamin D supplementation may be an additional therapeutic option for COVID-2019.
3.4. Vitamin E
As a powerful antioxidant, vitamin E is found to be effective in immune response and infection resistance in several trials [80]. This vitamin is a fat‐soluble vitamin. The prior reports have shown that it can play a vital role as an antioxidant to reduce oxidative stress by binding to free radicals [30,81]. The possible anti-viral effect of vitamin E in clinical trials involving small samples of children and adult people with Chronic hepatitis virus B (CHB) has been already recommended many years ago with highly interesting and promising outcomes [82]. It has been shown that there is a positive link between cell-mediated immune responses and plasma vitamin E according to the literature, while there is a negative tie between infection risks and plasma vitamin E in healthy people [83]. Clinical findings also suggest that vitamin E has a key role in respiratory tract infections. A study of randomized controlled trials of 617 caregivers found a decrement in risks of high-level respiratory tract infections. However, the results showed that there were not lower respiratory tract infections through routine supplementation for one year of 200 IU vitamin E intake [84]. Nevertheless, another study did not observe the incidence, period, or intensity of respiratory infections in the elderly community due to supplementary vitamin E (200 mg/day) [85]. In another study, the deficiency in vitamin E intensifies coxsackievirus B3 infection, in the myocardial cell in mice [86].
3.5. B vitamins
B-group vitamins are water‐soluble [30]. They are synthesized by yeasts, plants, and bacteria [87]. Each type of B vitamins has its own specific functions. For instance, vitamin B2 is a key element in the energy metabolism of all cells [88]. Keil et al. [89] reported that UV light and vitamin B2 can reduce the MERS‐CoV titer in human plasma products effectively. Vitamin B3 (Niacin) has anti-inflammatory effects and can play a significant role in maintaining immunological homeostasis [87]. In protein metabolism, vitamin B6 (pyridoxine) is also used and participates in over 100 reactions in body tissue [30]. Vitamins B12 and B6 and folate have been shown to support the activity of CD8 +cytotoxic T lymphocytes and natural killer cells, which is vital for antiviral protection [90]. Because B-group vitamins shortage can weaken host immune response, virus-infected patients need to be supplemented to improve the immune system’s function. B-group vitamins may, therefore, be selected as a basic alternative for COVID‐19 infection. The study by Gharote [91] suggested that the deficiency in vitamin B could weaken the host's immune response; thus, for virus-infected patients, the immune system should be enhanced by vitamin B supplements.
4. Trace elements’ roles in immune system function
Trace elements have a significant part in maintaining a healthy body [92]. A healthy balanced diet, rich in vitamins and trace elements will enhance the functioning of the immune system. Zinc, selenium, and copper also have favorable immune-modulating effects in viral respiratory infections among trace elements [18].
4.1. Zinc
In the growth and maintenance of immune cells, zinc is an essential trace element [7,93,94]. Zinc inhibits the RNA polymerase needed to replicate RNA viruses, such as those containing coronaviruses [85]. This indicates that zinc may be beneficial for host protection against RNA viruses. In addition, the deficiency of zinc impairs several aspects of innate immunity (e.g., respiratory burst and phagocytosis) [95]. Zinc supplementation in nursing elderly women at 30 mg/day increased serum zinc levels, which were mainly attributed to a rise in the number of T cells [96] associated with the enhancement of cell function. Zinc-deficient populations were also shown to be at increased threat of developing infections like HIV or hepatitis C virus (HCV) [93]. A clinically significant improvement in the zinc supplement group was found in 103 children (1 month to 5 years) compared with placebo [97].
Likewise, the supplement of zinc given to children with zinc deficiencies could reduce the morbidity associated with measles and mortality due to lower respiratory tract infections [98]. In addition, the rise in intracellular zinc with zinc ionophores such as pyrithione has been identified as a means of effectively impairing the replication of several RNA viruses (e.g., SARS coronavirus) [99]. As a result, zinc ionophore pyrrolidine dithiocarbamate [100] was used to inhibit flu virus replication in vitro and there are observations that zinc may inhibit the replication of SARS-CoVs virus in vitro as well [99]. Zinc is therefore considered as a possible supportive therapy for COVID-19 infection because of its effect on the immune-modulatory and its direct effect as antiviral [30]. In addition, the increasing chloroquine concentration of intracellular Zn2 + was supposed to mediate its anti-viral effect against SARS CoV-2 [101]. Thus, zinc supplements may affect not only symptoms associated with COVID‐19 (e.g., low respiratory tract infections and diarrhea), but also COVID‐19 itself.
4.2. Selenium
As a trace element, selenium has a large array of pleiotropic effects [102]. Selenium has an essential effect on the innate and acquired immune systems to optimize immune response. It plays a major role in the regulation of redox and antioxidant function and helps membrane integrity and protection of DNA damage [103]. Low selenium was linked to a raised mortality risk and poor immune function. Selenium supplementation has also presented antiviral influences [102]. A study by Broome et al. [104] has shown that increased intake of selenium in adults with marginal selenium concentration increases their immune function.
The dietary deficit of selenium inducing oxidative stress in host cells has also been reported to alter the virus genome that allows a virus to be a mild benign or moderate pathogenic virus [35,105]. Therefore, the selenium deficiency has been announced to not only raise the pathology of viral infections, but also contribute to genome changes in coxsackievirus, polio, and murine influenza viruses [[106], [107], [108], [109]]. Observations indicate that poor selenium condition may contribute to the production of more pathogenic virus strains, raising the risk and burden of viral infection, because selenium supports enzymes that along with vitamin E are beneficial to protect cells and tissues from oxidative damage by preventing the development of free radicals [105].
The supplementation of selenium has been effective in human immune response [110] and poliovirus reactions [104]. The supplementation of selenium may, therefore, be an efficient choice for treating the COVID‐19.
4.3. Copper
Copper is an important micronutrient for viral infections for both pathogens and hosts [111]. Farag, Baqi [112] found that copper is one of the most important human nutrients and demonstrated its role in numerous physiological processes including brain development and function and immune system function [113]. It has been demonstrated that copper plays an important role in immunity through involvement in the production and differentiation of immune cells like T-cell proliferation as well as natural killer activity and by metal sequestration machinery in the host [114,115]. Copper itself is also antimicrobial and promotes natural killer cell development, neutrophil, and monocyte and macrophage [7]. Copper deficiency is linked to changes in many copper status indices, including concentration of plasma coppers, ceruloplasmin and erythrocyte superoxide dismutase activities [116]. Sharma [117] reported that copper can be effective in the prevention of respiratory viruses, including those associated with SARS and MERS.
In another study by Wilson et al. [118], Mn, Cu, and Zn supplements have improved the clinical signs, performance, and TM balance of calves in response to the virus of bovine diarrhea or BVDV. Providing supplement Cu to calves that are marginally dietary in Cu-deficiency can increase levels of liver Cu during a respiratory challenge. The increased levels of Cu could positively affect the calves’ immune response during a combination of respiratory difficulties [118]. According to Rico, Roca-Botran [119], the results concluded that a replacement of copper may be a safe way to avoid or prevent resorption, thereby preventing osteoporosis. It is therefore important now more than ever to support the body in ensuring a healthy immune system response. A wise approach is to support the body in using an aggressive nutritional program using extremely efficient, carefully selected herbs, foods, and nutrients to support the whole body. Therefore, Cu supplementation and mineral deficiency correction may be beneficial for COVID-19 patients.
5. Summary and conclusion
This study conducted a bibliometric analysis of the published researches focusing on the potential impacts of vitamins and trace elements on COVID-19. The vitamins’ roles in the immune system and infectious disease were investigated. The result of the study demonstrated several outcomes based on the multiple analyses performed. As this study concentrated on presenting several visualizations of term-occurrences, keyword-occurrences, and citations-country visualization based on the published studies, future directions for researchers and decision-makers can be outlined.
As time is taken to develop effective vaccinations and antivirals, vitamins and other antioxidants are currently available to reduce the novel coronavirus-associated respiratory distress syndrome. All possible treatments must be carefully taken into account when combating COVID-19 infection. It is evident from the studies addressed in this review that recognizing the disease and its impact on the immune system would enhance the treatment of the disease. Based on the findings of previous studies, the immune system has essential protection against most infectious illnesses, including SARS-CoV-2. The main weapon against viral infections is a healthy immune system. The use of trace elements and vitamins is necessary for the normal function of the immune system. The research underlines the significance of daily diets of vitamins and trace elements for their possible inhibitory impact on viral disease. Overall, according to Group-1 and Group-2 of publications, the main findings of this review are as follows. First, considering the beneficial role of vitamins, a decrease in the levels of vitamins may lead to a poor immune system and accordingly lead to the infection of coronavirus. It also could be suggested that a moderate amount of vitamins can, therefore, be beneficial for preventing novel coronavirus at the early stage through improving the immune system. Hence, the outcomes are summarized as follows:
-
•
Vitamin A was found to be critical for the improvement of immune function. This vitamin is involved in the immune system’s development and is found to be important in the humoral immune processes and cellular immune responses. Furthermore, an enhanced immune response to influenza was observed after vitamin A supplementation. The reports showed that vitamin A can be effective for the prevention of lung infection as well.
-
•
Early use of vitamin C as an antioxidant can also be an effective treatment for patients with disease infection. As previous research has shown, under some conditions, vitamin C can be beneficial in preventing lower respiratory tract infection (LRTI). However, this must be further investigated through clinical studies. The findings of prior researches revealed that COVID-19 may cause infection of the respiratory tract and, therefore, under some conditions, vitamin C may be beneficial in preventing LRTI.
-
•
The findings also revealed that vitamin D and vitamin E supplementation may have a high potential to enhance SARS-CoV-2 resistance. Vitamin D is also found to have a major impact on the improvements of modulates adaptive immunity, cellular immunity, and expression of antioxidation-related genes. Further, according to the reports, vitamin D can stimulate the immune system (T cells and B cells) and accordingly be beneficial in the early stage of infection. This can accordingly suppress viral and bacterial infections.
-
•
The previous reports also suggest that vitamin B deficiency could weaken the host's immune response; thus, for virus-infected patients, the immune system should be enhanced by vitamin B supplements.
Second, we also investigated the role of trace elements in the immune system. They are micronutrients in our diet that are necessary to sustain optimal biological functions and optimal immune responses to infections including viral ones. It is clear from the above discussion that inadequate intake and status of trace elements can also lead to suppressed immunity. Since the immune system protects the host against pathogenic organisms (e.g., viruses, bacteria), the adequate intake and status of trace elements are necessary for infections caused by viruses. However, there is no underlying natural immunity that exists against COVID-19, it is, therefore, important to take care of nutritional habits at this time according to healthy and balanced nutritional patterns that contain plenty of minerals, antioxidants, and vitamins. Hence, considering Group-2 of publications, the beneficial outcomes of trace elements are summarized as follows:
-
•
Zinc is regarded as a potential supportive treatment for COVID-19 disease because of its influence on the immune-modulatory and its direct influence as antiviral [30].
-
•
Selenium supplementation has an effective influence on human immune response [110] and poliovirus reactions [104]. Hence, the supplementation of selenium may, therefore, be effective in reducing the risk of SARS-CoV-2 infection.
-
•
Cu supplementation and mineral deficiency correction may be beneficial for COVID-19 patients.
6. Recommendations and future work
Understanding the virus and its impacts on the immune system will allow providing suitable treatment policies and more efficient prevention methods [120]. The fact that particular sets of people are more vulnerable to serious infection and death reflects the difficulty of presenting appropriate treatment [9]. To rapidly manage and efficiently handle the viral infection, the innate immune system manages and concurrently activates the adaptive immune system. Hence, improving overall immunity is important for respiratory viral diseases (e.g., COVID-19) and this report based on the previous findings revealed that overall immunity can be improved through both trace elements and vitamins. Accordingly, the balanced intake of vitamins and trace elements may be beneficial for the prevention/treatment of infectious diseases such as influenza and COVID-19.
Despite doubts about the overall advantages of these vitamins and trace elements, many physicians started prescribing them regularly in the earlier time of the COVID-19 crisis. They argued that with so little knowledge about how to treat the disease and the safe usage of different vitamins and trace elements, there is an optimistic presumption that supplements might aid in the treatment of COVID-19 in its early stages. Thus, more clinical research on the role of trace elements and vitamins on the human immune system is needed to come up with a robust conclusion of the effectiveness of trace elements and vitamins for the prevention/treatment of COVID-19.
Although there are not enough proofs to suggest confidently adopting a specific dose of vitamins or trace elements for COVID-19, still doctors recommend that individuals need to get an appropriate amount of vitamins and trace elements to have a functioning immune system to combat the disease.
Declaration of Competing Interest
The authors of the study declare no conflict of interest.
Acknowledgements
This paper is partly supported by research grant with the vot number Q.J130000.2445.04G29.
References
- 1.Weiss S.R., Leibowitz J.L. Elsevier; 2011. Coronavirus Pathogenesis, in Advances in Virus Research; pp. 85–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su S., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hageman J.R. The coronavirus disease 2019 (COVID-19) Pediatr. Ann. 2020;49(3):e99–e100. doi: 10.3928/19382359-20200219-01. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N., et al. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020;92(4):408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F.-W., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delves P.J., Roitt I.M. The immune system. N. Engl. J. Med. 2000;343(1):37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 7.Calder P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prevent. Health. 2020 doi: 10.1136/bmjnph-2020-000085. p. bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sompayrac L.M. John Wiley & Sons; 2019. How the Immune System Works. [Google Scholar]
- 9.Nilashi M., et al. Can complementary and alternative medicines be beneficial in the treatment of COVID-19 through improving immune system function? J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., et al. Host immune response to influenza A virus infection. Front. Immunol. 2018;9:320. doi: 10.3389/fimmu.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich R.R., Chaplin D.D. Clinical Immunology. Elsevier; 2019. The human immune response. p. 3-17. e1. [Google Scholar]
- 13.Li G., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel N., et al. Baseline serum vitamin A and D levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019;11(10):907. doi: 10.3390/v11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iovino L., et al. High-dose zinc oral supplementation after stem cell transplantation causes an increase of TRECs and CD4+ naive lymphocytes and prevents TTV reactivation. Leuk. Res. 2018;70:20–24. doi: 10.1016/j.leukres.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Ivory K., et al. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr. 2017;36(2):407–415. doi: 10.1016/j.clnu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saul A.W. 2020. Vitamin C Protects Against Coronavirus. [Google Scholar]
- 18.Jayawardena R., et al. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson A., et al. Effect of fruit and vegetable consumption on immune function in older people: a randomized controlled trial. Am. J. Clin. Nutr. 2012;96(6):1429–1436. doi: 10.3945/ajcn.112.039057. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard A. Statistical bibliography or bibliometrics. J. Doc. 1969;25(4):348–349. [Google Scholar]
- 21.Khalil G.M., Crawford C.A.G. A bibliometric analysis of US-based research on the behavioral risk factor surveillance system. Am. J. Prev. Med. 2015;48(1):50–57. doi: 10.1016/j.amepre.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobo M.J., et al. Science mapping software tools: review, analysis, and cooperative study among tools. J. Am. Soc. Inf. Sci. Technol. 2011;62(7):1382–1402. [Google Scholar]
- 24.Van Eck N.J., et al. A comparison of two techniques for bibliometric mapping: multidimensional scaling and VOS. J. Am. Soc. Inf. Sci. Technol. 2010;61(12):2405–2416. [Google Scholar]
- 25.Heersmink R., et al. Bibliometric mapping of computer and information ethics. Ethics Inf. Technol. 2011;13(3):241. [Google Scholar]
- 26.Flis I., van Eck N.J. Framing psychology as a discipline (1950–1999): a large-scale term co-occurrence analysis of scientific literature in psychology. Hist. Psychol. 2018;21(4):334. doi: 10.1037/hop0000067. [DOI] [PubMed] [Google Scholar]
- 27.Jun S.-P., Yoo H.S., Choi S. Ten years of research change using Google Trends: from the perspective of big data utilizations and applications. Technol. Forecast. Soc. Change. 2018;130:69–87. [Google Scholar]
- 28.Rodrigues S., et al. Mapping patient safety: a large-scale literature review using bibliometric visualisation techniques. BMJ Open. 2014;4(3) doi: 10.1136/bmjopen-2013-004468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggini S., et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007;98(S1):S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCullough F., Northrop-Clewes C., Thurnham D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999;58(2):289–293. doi: 10.1017/s0029665199000403. [DOI] [PubMed] [Google Scholar]
- 32.Aluisio A.R., et al. Vitamin A supplementation was associated with reduced mortality in patients with Ebola virus disease during the West African outbreak. J. Nutr. 2019;149(10):1757–1765. doi: 10.1093/jn/nxz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reifen R. Vitamin A as an anti-inflammatory agent. Proc. Nutr. Soc. 2002;61(3):397–400. doi: 10.1079/PNS2002172. [DOI] [PubMed] [Google Scholar]
- 34.Wiedermann U., et al. Vitamin A deficiency increases inflammatory responses. Scand. J. Immunol. 1996;44(6):578–584. doi: 10.1046/j.1365-3083.1996.d01-351.x. [DOI] [PubMed] [Google Scholar]
- 35.Guillin O.M., et al. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jee J., et al. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am. J. Vet. Res. 2013;74(10):1353–1362. doi: 10.2460/ajvr.74.10.1353. [DOI] [PubMed] [Google Scholar]
- 37.Villamor E., et al. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics. 2002;109(1) doi: 10.1542/peds.109.1.e6. p. e6-e6. [DOI] [PubMed] [Google Scholar]
- 38.Semba R.D. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999;58(3):719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- 39.Owusu-Agyei S., et al. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr. J. 2013;12(1):131. doi: 10.1186/1475-2891-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okafor F. Vitamin A supplementation of malaria-infected pregnant women and infant birth weight outcomes a case study of Ebonyi State. Nigeria. Gastro. 2014;2:109. [Google Scholar]
- 41.Campa A., et al. The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana. Nutr. Diet. Suppl. 2017;2017(9):37. doi: 10.2147/NDS.S123545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kańtoch M., et al. Importance of vitamin A deficiency in pathology and immunology of viral infections. Rocz. Panstw. Zakl. Hig. 2002;53(4):385–392. [PubMed] [Google Scholar]
- 43.Trottier C., et al. Retinoids inhibit measles virus through a type I IFN-dependent bystander effect. Faseb J. 2009;23(9):3203–3212. doi: 10.1096/fj.09-129288. [DOI] [PubMed] [Google Scholar]
- 44.Patel N., et al. Baseline serum vitamin a and d levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019;11(10):907. doi: 10.3390/v11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West C.E., et al. Epithelia-damaging virus infections affect vitamin A status in chickens. J. Nutr. 1992;122(2):333–339. doi: 10.1093/jn/122.2.333. [DOI] [PubMed] [Google Scholar]
- 46.Galanakis C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9(4):523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carr A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care. 2020;24(1):1–2. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y., et al. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013;13(2):70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jariwalla R.J., Harakeh S. Springer; 1996. Antiviral and Immunomodulatory Activities of Ascorbic Acid, in Subcellular Biochemistry; pp. 215–231. [PubMed] [Google Scholar]
- 50.Byun S.H., Jeon Y. Administration of vitamin C in a patient with herpes zoster-a case report. Korean J. Pain. 2011;24(2):108. doi: 10.3344/kjp.2011.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harakeh S., Jariwalla R.J., Pauling L. Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. Proc. Natl. Acad. Sci. 1990;87(18):7245–7249. doi: 10.1073/pnas.87.18.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez M.J., et al. High dose vitamin C and influenza: a case report. J. Orthomol. Med. 2018;33(3) [Google Scholar]
- 53.Zarubaev V., et al. Protective activity of ascorbic acid at influenza infection. INFEKTSIYA I IMMUNITET. 2017;7(4):319–326. [Google Scholar]
- 54.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemilä H. Vitamin C and SARS coronavirus. J. Antimicrob. Chemother. 2003;52(6):1049–1050. doi: 10.1093/jac/dkh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atherton J., Kratzing C., Fisher A. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978;56(3):195–199. doi: 10.1007/BF01317848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truwit J.D., et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. Jama. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim W.-Y., et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score-based analysis of a before-after cohort study. J. Crit. Care. 2018;47:211–218. doi: 10.1016/j.jcrc.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Liu K., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19) Medicine in Drug Discovery. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grant W.B., et al. 2020. Vitamin D Supplementation Could Prevent and Treat Influenza, Coronavirus, and Pneumonia Infections. [Google Scholar]
- 63.Gombart A.F. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang T.-T., et al. Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 65.Zdrenghea M.T., et al. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017;27(1):e1909. doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 66.Martínez-Moreno J., Hernandez J.C., Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol. Cell. Biochem. 2020;464(1–2):169–180. doi: 10.1007/s11010-019-03658-w. [DOI] [PubMed] [Google Scholar]
- 67.Arboleda J.F., Urcuqui-Inchima S. Vitamin D-regulated MicroRNAs: are they protective factors against dengue virus infection? Adv. Virol. 2016;2016 doi: 10.1155/2016/1016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarez N., Aguilar-Jimenez W., Rugeles M.T. The potential protective role of vitamin D supplementation on HIV-1 infection. Front. Immunol. 2019;10:2291. doi: 10.3389/fimmu.2019.02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goncalves-Mendes N., et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front. Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ginde A.A., et al. High‐dose monthly vitamin D for prevention of acute respiratory infection in older long‐term care residents: a randomized clinical trial. J. Am. Geriatr. Soc. 2017;65(3):496–503. doi: 10.1111/jgs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wimalawansa S.J. Global epidemic of coronavirus—Covid-19: what can we do to minimize risks. European Journal of Biomedical. 2020;7(3):432–438. [Google Scholar]
- 72.Grant W.B., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nonnecke B., et al. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infection is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J. Dairy Sci. 2014;97(9):5566–5579. doi: 10.3168/jds.2014-8293. [DOI] [PubMed] [Google Scholar]
- 74.Lei G.-S., et al. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob. Agents Chemother. 2017;61(10):e01226–17. doi: 10.1128/AAC.01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mousavi S., Bereswill S., Heimesaat M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. (Bp) 2019;9(3):73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biancatelli R.M.L.C., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev. Anti. Ther. 2020;18(2):99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 77.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020:1. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCartney D., Byrne D. Optimisation of vitamin D status for enhanced Immuno-protection against Covid-19. Ir. Med. J. 2020;113(4):58. [PubMed] [Google Scholar]
- 79.Alipio M. 2020. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected With Coronavirus-2019 (COVID-19) Available at SSRN 3571484. [Google Scholar]
- 80.Elenkov I.J., Chrousos G.P. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metab. 1999;10(9):359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 81.Galmés S., Serra F., Palou A. Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances. Nutrients. 2018;10(12):1919. doi: 10.3390/nu10121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiorino S., et al. 2020. The Rationale for a Multi-step Therapeutic Approach Based on Antivirals and Drugs With Immunomodulatory Activity in Patients With Coronavirus-Sars2-Induced Disease of Different Severity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chavance M., et al. Vitamin status, immunity and infections in an elderly population. Eur. J. Clin. Nutr. 1989;43(12):827–835. [PubMed] [Google Scholar]
- 84.Meydani S.N., et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. Jama. 2004;292(7):828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graat J.M., Schouten E.G., Kok F.J. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. Jama. 2002;288(6):715–721. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 86.Beck M.A., et al. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J. Nutr. 1994;124(3):345–358. doi: 10.1093/jn/124.3.345. [DOI] [PubMed] [Google Scholar]
- 87.Yoshii K., et al. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Powers H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003;77(6):1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 89.Keil S.D., Bowen R., Marschner S. Inactivation of M iddle E ast respiratory syndrome coronavirus (MERS‐C o V) in plasma products using a riboflavin‐based and ultraviolet light‐based photochemical treatment. Transfusion. 2016;56(12):2948–2952. doi: 10.1111/trf.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tamura J., et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12‐deficient patients by methyl‐B12 treatment. Clin. Exp. Immunol. 1999;116(1):28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gharote M.A. Role of poly (ADP) ribose polymerase-1 inhibition by nicotinamide as a possible additive treatment to modulate host immune response and prevention of cytokine storm in COVID-19. Indian J. Med. Sci. 2020;72(1):25. [Google Scholar]
- 92.Skalnaya M.G., Skalny A.V. Publishing House of Tomsk State University; Tomsk: 2018. Essential Trace Elements in Human Health: a Physician’s View. [Google Scholar]
- 93.Read S.A., et al. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erickson K.L., Medina E.A., Hubbard N.E. Micronutrients and innate immunity. J. Infect. Dis. 2000;182(Supplement_1):S5–S10. doi: 10.1086/315922. [DOI] [PubMed] [Google Scholar]
- 96.Barnett J.B., et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016;103(3):942–951. doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- 97.Acevedo-Murillo J.A., et al. Zinc supplementation promotes a Th1 response and improves clinical symptoms in less hours in children with pneumonia younger than 5 years old. A randomized controlled clinical trial. Front. Pediatr. 2019;7:431. doi: 10.3389/fped.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Awotiwon A.A., et al. Zinc supplementation for the treatment of measles in children. Cochrane Database Syst. Rev. 2017;6 doi: 10.1002/14651858.CD011177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Te Velthuis A.J., et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uchide N., et al. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002;56(3):207–217. doi: 10.1016/s0166-3542(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 101.Skalny A.V., et al. Zinc and respiratory tract infections: perspectives for COVID‑19. Int. J. Mol. Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 103.Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007;51(4):301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 104.Broome C.S., et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004;80(1):154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 105.Harthill M. Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011;143(3):1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beck M.A., et al. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat. Med. 1995;1(5):433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 107.Beck M.A., Levander O.A. Host nutritional status and its effect on a viral pathogen. J. Infect. Dis. 2000;182(Supplement_1):S93–S96. doi: 10.1086/315918. [DOI] [PubMed] [Google Scholar]
- 108.Nelson H.K., et al. Host nutritional selenium status as a driving force for influenza virus mutations. Faseb J. 2001;15(10):1846–1848. [PubMed] [Google Scholar]
- 109.Beck M.A., et al. Selenium deficiency increases the pathology of an influenza virus infection. Faseb J. 2001;15(8):1481–1483. [PubMed] [Google Scholar]
- 110.Hawkes W.C., Kelley D.S., Taylor P.C. The effects of dietary selenium on the immune system in healthy men. Biol. Trace Elem. Res. 2001;81(3):189–213. doi: 10.1385/BTER:81:3:189. [DOI] [PubMed] [Google Scholar]
- 111.Raha S., et al. Is Copper beneficial for COVID-19 patients? Med. Hypotheses. 2020:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farag H.A., et al. The role of nutrients in supporting the immune system against viral infection; newly emerged coronavirus (COVID-19): a narrative review. Kurdistan J. Appl. Res. 2020:84–96. [Google Scholar]
- 113.Taylor A.A., et al. Critical review of exposure and effects: implications for setting regulatory health criteria for ingested copper. Environ. Manage. 2020;65(1):131–159. doi: 10.1007/s00267-019-01234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li C., Li Y., Ding C. The role of copper homeostasis at the host-pathogen 634 Axis: from Bacteria to Fungi. Int. J. Mol. Sci. 2019;635(20):1. doi: 10.3390/ijms20010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gombart A., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1) doi: 10.3390/nu12010236. pii: E236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turnlund J.R., et al. Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am. J. Clin. Nutr. 2004;79(6):1037–1044. doi: 10.1093/ajcn/79.6.1037. [DOI] [PubMed] [Google Scholar]
- 117.Sharma L. Dietary management to build adaptive immunity against COVID-19. Journal of PeerScientist. 2020;2(2):e1000016. [Google Scholar]
- 118.Wilson B., et al. Effect of copper, manganese, and zinc supplementation on the performance, clinical signs, and mineral status of calves following exposure to bovine viral diarrhea virus type 1b and subsequent Mannheimia haemolytica infection. J. Anim. Sci. 2016;94(3):1123–1140. doi: 10.2527/jas.2015-9503. [DOI] [PubMed] [Google Scholar]
- 119.Rico H., et al. The effect of supplemental copper on osteopenia induced by ovariectomy in rats. Menopause. 2000;7(6):413–416. doi: 10.1097/00042192-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 120.Raj R.S., Bonney E.A., Phillippe M. Influenza, immune system, and pregnancy. Reprod. Sci. 2014;21(12):1434–1451. doi: 10.1177/1933719114537720. [DOI] [PMC free article] [PubMed] [Google Scholar]