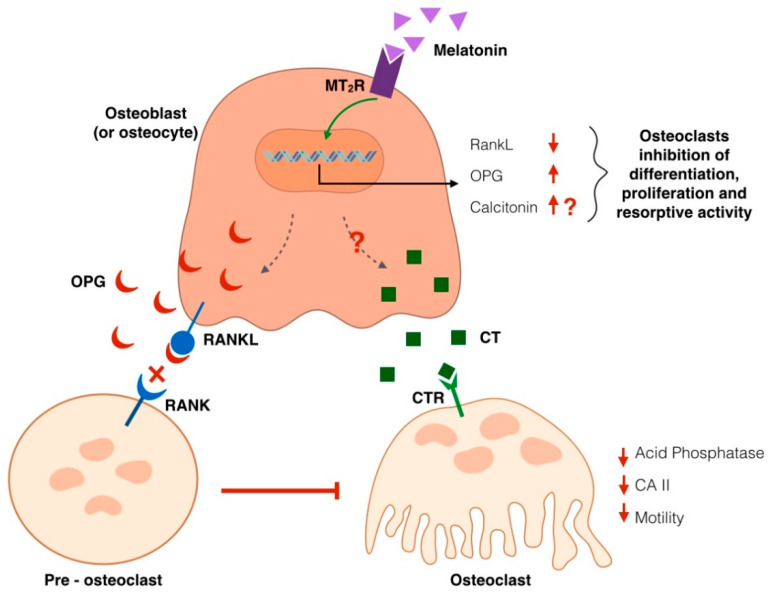
Figure 2.
Molecular mechanism proposed for melatonin’s antiresorptive effect on bone density. Melatonin binding to its G-protein coupled receptor (MT2R), located on the cell membrane of osteoblasts/osteocytes and mesenchymal cells, induces a signaling cascade culminating in the modulation of genes that influence osteoclast differentiation and activity; specifically, a downregulation of RANKL and upregulation of osteoprotegerin (OPG), a decoy receptor produced by stromal cells as negative feedback to control osteoclastogenesis. Melatonin has also been proposed to produce an upregulation of calcitonin, whose binding to its receptor (calcitonin binding receptor or CTR) on osteoclasts induces a rapid cell contraction, causes inhibition of osteoclast motility. Calcitonin also inhibits (red arrows) other pathways associated with osteoclast activity, such as the release of acid phosphatase and the expression of carbonic anhydrase II (CA II), a cytosolic enzyme involved in the maintenance of an acidic environment, necessary for osteoclast resorption.