Figure 3.
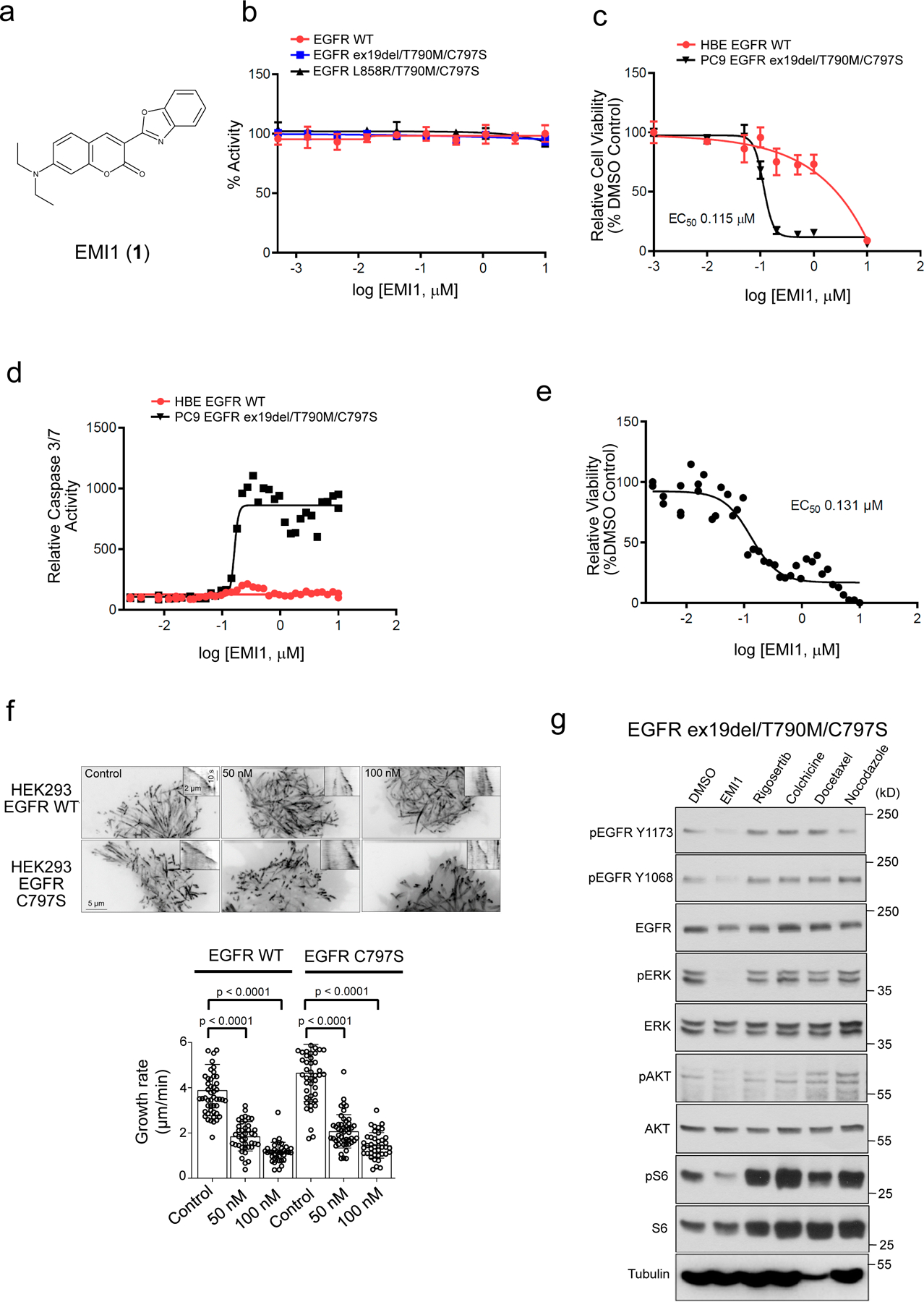
Validation of EMI1 as an EGFR ex19del/T790M/C797S and EGFR L858R/T790M/C797S activating mutant inhibitor. (a) Chemical structure for EMI1. (b) in vitro kinase assay of recombinant kinase domain (residues 696–1022) of indicated mutant or WT EGFR in the presence of EMI1. Results are shown as the average ± SD for two independent experiments. (c) Effect of EMI1 on the viability of PC9 EGFR ex19del/T790M/C797S and HBE bronchial epithelial lung EGFR WT control cells. Results are shown as the average ± SD for three independent experiments (d) Effect of EMI1 on caspase 3/7 activity in PC9 EGFR ex19del/T790M/C797S and HBE EGFR WT cells. Results are shown as single 36-point dose response experiments. (e) Viability assay measuring effect of EMI1 on PC9 EGFR ex19del/T790M/C797S organoid growth. Results are shown as single 36-point dose response experiments. (f) Maximum intensity projections (stream acquisition/exposure time 500 ms/100 frames) showing the effect of EMI1 on microtubule dynamics in HEK293 MaMTH reporter cells stably expressing EGFR WT or EGFR L858R/T790M/C797S transfected with EB3-TagRFP as a microtubule plus end marker. The contrast is inverted. Graph shows quantification of microtubule plus end velocity in HEK293 reporter cells for EMI1. n = 51, 41, 36 for HEK293 EGFR WT, control, 50 and 100 nM. n = 49, 47, 41 for HEK293 EGFR C797S control, 50 and 100 nM. Significant p-values are displayed and were calculated using the Mann-Whitney test. (g) Western blot analysis showing activity of EMI1 and other microtubule targeting compounds after 2 hours treatment on EGFR ex19del/T790M/C797S activation and downstream signalling in PC9 triple mutant cells (see Supplementary Fig. 25 for source blot images). Results are representative of at least two independent experiments.
