Abstract
Background
Only high‐risk tumors with extranodal extension (ENE) and/or positive surgical margins (PSM) benefit from adjuvant therapy (AT) with concurrent chemoradiation (CRT) compared to radiation therapy (RT) in locally advanced head and neck squamous cell carcinoma (HNSCC). Optimal treatment for intermediate‐risk tumors remains controversial. We categorized patients based on their surgical pathologic risk factors and described AT treatment patterns and associated survival outcomes.
Methods
Patients were identified from CHANCE, a population‐based study, and risk was classified based on surgical pathology review. High‐risk patients (n = 204) required ENE and/or PSM. Intermediate‐risk (n = 186) patients had pathological T3/T4 disease, perineural invasion (PNI), lymphovascular invasion (LVI), or positive lymph nodes without ENE. Low‐risk patients (n = 226) had none of these features.
Results
We identified 616 HPV‐negative HNSCC patients who received primary surgical resection with neck dissection. High‐risk patients receiving AT had favorable OS (HR 0.50, p = 0.013) which was significantly improved with the addition of chemotherapy compared to RT alone (HR 0.47, p = 0.021). When stratified by node status, the survival benefit of AT in high‐risk patients persisted only among those who were node‐positive (HR: 0.17, p < 0.0005). On the contrary, intermediate‐risk patients did not benefit from AT (HR: 1.26, p = 0.380) and the addition of chemotherapy was associated with significantly worse OS compared to RT (HR: 1.76, p = 0.046).
Conclusion
In high‐risk patients, adjuvant chemoradiotherapy improved OS compared to RT alone. The greatest benefit was in node‐positive cases. In intermediate‐risk patients, the addition of chemotherapy to RT increased mortality risk and therefore should only be used cautiously in these patients.
Keywords: adjuvant radiotherapy, chemotherapy, head and neck cancer, head and neck squamous cell carcinoma, HPV‐negative, risk factors, survival
The addition of chemotherapy to adjuvant radiotherapy could improve survival. This is observed only among high‐risk patients. When stratified by nodal status, the benefit exists only in node‐positive patients. In intermediate‐risk patients chemotherapy addition decreases survival. Risk categorization is important for treatment selection and survival outcomes.
![]()
1. INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide. 1 It constitutes a family of epithelial malignancies including oral cavity, oropharyngeal, and laryngeal squamous cell carcinomas. 2 Despite improvement in therapeutic options, 5‐year overall survival (OS) has remained relatively unchanged at around 50–60%. 3 , 4 , 5 Historically, TNM stage has been the most important predictor of OS. 5 However, several reports have identified that clinical (nodal disease in levels IV and V) and pathological factors (extranodal extension, ENE; positive surgical margins, PSM; perineural invasion, PNI, and lymphovascular invasion, LVI) affect prognosis and predict poor locoregional control, worse OS, and worse relapse‐free survival (RFS). 6 , 7 , 8 , 9 Notably, the 8th AJCC/UICC edition implemented ENE in the nodal (N)‐category of TNM staging. 10
Therapeutically, primary surgical resection with or without adjuvant therapy remains a standard of care option for patients with HNSCC. 7 In the phase III EORTC 22391 and RTOG 95–01 trials, HNSCC patients with ENE and/or PSM had improved survival outcomes following adjuvant cisplatin‐based CRT compared to adjuvant radiotherapy (aRT) alone 11 , 12 , 13 , 14 and thus has become standard of care treatment practice. Furthermore, adjuvant chemoradiation (aCRT) is suggested for all patients with adverse risk features (ARF), including ENE, PSM, PNI, nodal disease in levels IV and V, LVI). 7 However, in patients without ARFs, there is no consensus for use in the adjuvant setting. In two National Cancer Database studies of HNSCC patients, the use of aCRT did not confer a survival benefit in patients without positive margins or ENE. 15 , 16 One of the studies identified an increasing survival benefit associated with the extent of nodal involvement. 16 Given the significant adverse events associated with aCRT and the impact on quality of life (QoL), optimizing the selection of adjuvant therapy (AT) is essential to maximize survival and minimize treatment‐associated adverse outcomes. 17 , 18
We sought to address the impact of AT on the survival of HPV‐negative HNSCC patients in a large population‐based database from North Carolina. We used an ARF‐based patient classification scheme described by the National Comprehensive Cancer Network (NCCN) and commonly cited literature. 7 , 19 , 20 , 21 High risk included only patients with ENE and/or PSM. Intermediate‐risk was defined as pathological T3/T4 disease, PNI, LVI, or positive lymph nodes without ENE or PSM. Patients without an ARF were defined as low‐risk.
2. METHODS
2.1. Study Population
Patients diagnosed with HPV‐negative HNSCC were identified from the Carolina Head and Neck Cancer Epidemiology Study (CHANCE), a population‐based study in North Carolina. 22 Cases were eligible to participate in CHANCE if they had been diagnosed with a first primary squamous cell carcinoma of the oral cavity, pharynx, or larynx between January 1, 2002, and February 28, 2006; aged 20 to 80 years at diagnosis; and resided in a 46‐county region in central North Carolina. The study was approved by the Institutional Review Board (IRB) of all participating institutions.
For each patient who received primary surgical resection, medical records were retrospectively reviewed and the following data were collected: age, sex, tumor site, surgical treatment category (surgical resection only, surgical resection and aRT, surgical resection and aCRT), HPV tumor status, LVI, PNI, ENE, T stage, and number of positive lymph nodes. HPV tumor status was evaluated using p16 immunohistochemistry and was performed by the International Agency for Research on Cancer (IARC). The full p16 immunohistochemistry protocol has been previously described. 23 For the purpose of this study, patients with p16‐positive oropharyngeal cancer were excluded, as HPV‐associated HNSCC had a distinct biological behavior and more favorable prognosis. Information on individual behaviors (tobacco and alcohol use) and socioeconomic status (household income, education, and insurance status) was collected through in‐home interviews by trained nurse interviewers during the creation of CHANCE. Race was self‐identified from an interview question.
Staging classification was based on the American Joint Commission on Cancer (AJCC) 7th edition, which was available at the time of data collection.
2.2. Statistical Analysis
Descriptive statistics were calculated, and bivariate testing methods included chi‐square and Fisher's exact tests. An alpha criterion of p ≤ 0.05 was used for all significance testing. CHANCE data were linked to the National Death Index (NDI) based on name, social security number, date of birth, sex, race, and state of residence to identify deaths through December 31, 2013. Overall survival (OS) was estimated using hazard ratios (HR) and corresponding 95% confidence intervals (CI) obtained via Cox regression models stratified by adverse risk group. Models were examined before and after adjusting for the following covariates: tumor site, age, sex, race, smoking, alcohol, TNM stage, treatment, and pathologic risk group Stata 15.1 (StataCorp LP, College Station, TX) was used for all statistical analyses.
3. RESULTS
3.1. Baseline Characteristics By Risk Category
We identified 616 HPV‐negative HNSCC patients who received primary surgical resection and were classified as either low‐risk (n = 226), intermediate‐risk (n = 186), or high‐risk (n = 204) based on pathological outcomes (Table 1). The majority of patients were male (72%) and identified as white race (72%). Intermediate‐ and high‐risk patients were more likely to be male, lack medical insurance, have a household income < $20,000, use tobacco, and drink alcohol. Intermediate‐risk and high‐risk patients had lower socioeconomic status compared to patients classified as low‐risk. As expected in an HPV‐negative population, most patients in the cohort had drinking (84%) defined as >1 drink per week and heavy smoking histories (78%) defined as >10 pack‐years.
TABLE 1.
Demographic characteristics of HPV‐negative HNSCC patients by risk category
| Pathologic risk category | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Risk ( n = 226) | Intermediate Risk (n = 186) | High Risk ( n = 204) | Total ( n = 616) | p‐value | |||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age | 0.281 | ||||||||
| <50 | 42 | 19 | 43 | 23 | 43 | 21 | 128 | 21 | |
| 50–65 | 102 | 45 | 92 | 49 | 87 | 43 | 281 | 46 | |
| >65 | 82 | 36 | 51 | 27 | 74 | 36 | 207 | 34 | |
| Sex | <0.005 | ||||||||
| Male | 142 | 63 | 137 | 74 | 163 | 80 | 442 | 72 | |
| Female | 84 | 37 | 49 | 26 | 41 | 20 | 174 | 28 | |
| Race | 0.062 | ||||||||
| White | 179 | 79 | 127 | 68 | 140 | 69 | 446 | 72 | |
| Black | 43 | 19 | 56 | 30 | 61 | 30 | 160 | 26 | |
| Other | 4 | 2 | 3 | 2 | 3 | 1 | 10 | 2 | |
| Education | 0.001 | ||||||||
| Less Than High School | 63 | 28 | 61 | 33 | 86 | 42 | 210 | 34 | |
| High School Grad | 57 | 25 | 60 | 32 | 60 | 29 | 177 | 29 | |
| Greater than High School | 106 | 47 | 65 | 35 | 58 | 28 | 229 | 37 | |
| Insurance Category | 0.025 | ||||||||
| Private | 81 | 39 | 60 | 33 | 65 | 33 | 206 | 35 | |
| Medicaid/Medicare | 71 | 34 | 61 | 34 | 78 | 39 | 210 | 36 | |
| None | 15 | 7 | 34 | 19 | 22 | 11 | 71 | 12 | |
| Other | 40 | 19 | 27 | 15 | 34 | 17 | 101 | 17 | |
| Income | 0.001 | ||||||||
| Greater than 50 k | 66 | 31 | 46 | 26 | 50 | 26 | 162 | 28 | |
| 20 k–50 k | 91 | 43 | 47 | 27 | 68 | 35 | 206 | 35 | |
| <20 k | 56 | 26 | 81 | 47 | 78 | 40 | 215 | 37 | |
| Smoking History | 0.016 | ||||||||
| <10 Pack Years | 63 | 28 | 38 | 21 | 34 | 17 | 135 | 22 | |
| >10 Pack Years | 162 | 72 | 146 | 79 | 170 | 83 | 478 | 78 | |
| Drinking History | 0.018 | ||||||||
| <1 Drink / Week | 47 | 21 | 20 | 11 | 31 | 16 | 98 | 16 | |
| >1 Drink / Week | 176 | 79 | 166 | 89 | 166 | 84 | 508 | 84 | |
3.2. Pathologic and Treatment Characteristics
The larynx/hypopharynx (35%), oral cavity (27%), and oropharynx (12%) were the most common primary tumor sites. Most patients receiving AT (n = 282, 89%) were classified as intermediate‐ or high‐risk (p < 0.0005). Among those, 68% (n = 191) received aRT, whereas 32% (n = 91) received aCRT. In the high‐risk group, 79% (n = 158) had PSM, whereas 86% (n = 59) had ENE. Interestingly, only 26% (n = 53) of high‐risk patients received aCRT whereas 50% (n = 103) received aRT. Among intermediate‐risk patients, 20% (n = 38) received aCRT, 47% (n = 88) received aRT, and 32% (n = 60) had no AT. We describe post‐resection pathologic characteristics in detail according to ARF classification (Table 2).
TABLE 2.
Post‐resection pathological and treatment characteristics of HNSCC patients by risk category
| Pathologic Risk Category | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low risk | Intermediate risk | High risk | Total | ||||||
| No. | % | No. | % | No. | % | No. | % | p‐value | |
| Tumor Site | <0.0005 | ||||||||
| Oral cavity | 80 | 35 | 42 | 23 | 45 | 22 | 167 | 27 | |
| Oropharynx | 15 | 7 | 27 | 15 | 34 | 17 | 76 | 12 | |
| Larynx/Hypopharynx | 53 | 23 | 69 | 37 | 94 | 46 | 216 | 35 | |
| NOS | 78 | 35 | 48 | 26 | 31 | 15 | 157 | 25 | |
| Surgical Margin | <0.0005 | ||||||||
| Negative | 186 | 100 | 158 | 100 | 43 | 21 | 387 | 71 | |
| Positive | 0 | 0 | 0 | 0 | 158 | 79 | 158 | 29 | |
| Extranodal Extension | <0.0005 | ||||||||
| Negative | 5 | 100 | 48 | 100 | 10 | 14 | 63 | 52 | |
| Positive | 0 | 0 | 0 | 0 | 59 | 86 | 59 | 48 | |
| T‐Stage | <0.0005 | ||||||||
| Early stage (1–2) | 226 | 100 | 67 | 36 | 133 | 65 | 426 | 69 | |
| Advanced stage (3–4) | 0 | 0 | 119 | 64 | 71 | 35 | 190 | 31 | |
| Lymphovascular Invasion | <0.0005 | ||||||||
| No | 159 | 95 | 117 | 81 | 145 | 77 | 421 | 84 | |
| Yes | 8 | 5 | 28 | 19 | 43 | 23 | 79 | 16 | |
| N‐Stage | <0.0005 | ||||||||
| N‐negative | 226 | 100 | 59 | 32 | 104 | 51 | 389 | 63 | |
| N‐positive | 0 | 0 | 127 | 68 | 100 | 49 | 227 | 37 | |
| Surgical Treatment Category | <0.0005 | ||||||||
| Surgery Only | 192 | 85 | 60 | 32 | 48 | 24 | 300 | 49 | |
| Surgery + RT | 33 | 15 | 88 | 47 | 103 | 50 | 224 | 36 | |
| Surgery + CRT | 1 | 0 | 38 | 20 | 53 | 26 | 92 | 15 | |
3.3. Overall survival by pathologic risk category
There were significant differences in overall survival among HPV‐negative HNSCC patients classified by risk. The crude 5‐year OS rate was 73% for low‐risk patients, 55% for intermediate‐risk patients, and 52% for high‐risk patients (Figure 1).
FIGURE 1.
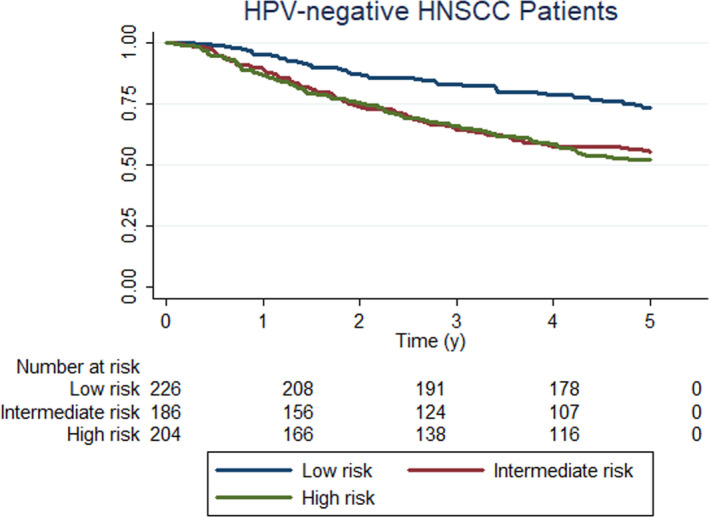
Unadjusted overall survival curves for HPV‐negative HNSCC patients according to pathologic risk category
Patients were stratified by pathological risk category to examine the associations between treatment modality and OS (Table 3 and Figure 2A‐D). All models adjusted for tumor site, age, sex, race, smoking, alcohol, and TNM stage. Among high‐risk patients, those receiving AT had significantly better OS compared to surgery alone (HR 0.50, 95% CI 0.29–0.86, p = 0.013). When stratified by adjuvant therapy, aCRT offered significant survival benefit compared to surgery alone (HR 0.30, 95% CI 0.15–0.61, p = 0.001) and compared to surgery plus aRT (HR 0.47, 95% CI 0.25 – 0.89, p = 0.021). Among intermediate‐risk patients, those receiving AT did not show survival benefit compared to surgery alone (HR 1.26, 95%CI: 0.75–2.10, p = 0.380). When stratified by adjuvant therapy, aCRT was associated with worse OS compared to aRT (HR: 1.76, 95%CI 1.01 – 3.05, p = 0.046) and surgery alone (HR 1.86, 95%CI 1.00–3.44, p = 0.050). Low‐risk patients receiving aRT (HR 2.01, 95% CI 0.95–4.25; p = 0.068) had no significant difference in OS compared to surgery alone.
TABLE 3.
Association of stage and treatment with overall survival in HPV‐negative HNSCC patients
| Low risk | Intermediate risk | High risk | ||||
|---|---|---|---|---|---|---|
| Cox regression model a | HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value |
| TNM Classification | ||||||
| I | reference | — | — | reference | ||
| II | 1.48 (0.84 – 2.60) | 0.173 | — | — | 1.02 (0.44 – 2.32) | 0.970 |
| III | — | reference | 1.90 (0.75 – 4.83) | 0.178 | ||
| IV | — | 1.39 (0.84 – 2.28) | 0.198 | 3.62 (1.97 – 6.65) | <0.0005 | |
| Treatment | ||||||
| Surgery | reference | reference | reference | |||
| Surgery + Radiotherapy | 2.01 (0.95 – 4.25) | 0.068 | 1.06 (0.61–1.83) | 0.846 | 0.60 (0.34–1.07) | 0.083 |
| Surgery + Chemoradiotherapy | — | — | 1.86 (1.00–3.44) | 0.050 | 0.30 (0.15–0.61) | 0.001 |
The numbers of low risk patients receiving surgery + chemoradiotherapy were insufficient for survival analysis.
Abbreviations: CI, Confidence intervals; HR, Hazard ratio.
Estimates obtained from multivariable Cox regression modeling including terms for tumor site, age, sex, race, smoking, alcohol, TNM stage (AJCC 7th Edition), treatment and pathologic risk group.
FIGURE 2.
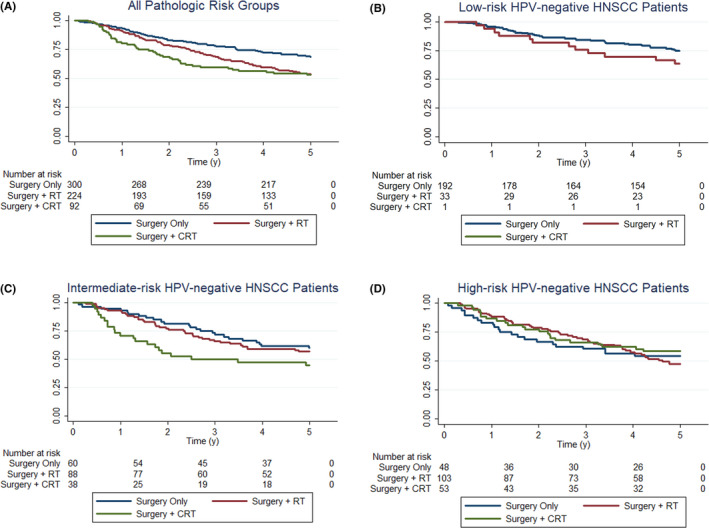
Unadjusted overall survival curves for all HPV‐negative HNSCC patients according to treatment via surgery vs. surgery and radiotherapy vs. surgery and chemoradiotherapy (A), low‐risk HPV‐negative HNSCC patients according to treatment via surgery vs. surgery and radiotherapy vs. surgery and chemoradiotherapy (B), intermediate‐risk HPV‐negative HNSCC patients according to treatment via surgery vs. surgery and radiotherapy vs. surgery and chemoradiotherapy (C), high‐risk HPV‐negative HNSCC patients according to treatment via surgery vs. surgery and radiotherapy vs. surgery and chemoradiotherapy (D)
Next, we performed a subset analysis for OS stratified by both pathologic risk category and nodal status (positive vs. negative). We found that the survival benefit of AT among high‐risk patients was present only in the node‐positive group (HR 0.17, 95% CI 0.07–0.41, p < 0.0005), and this association persisted when evaluating aRT (HR 0.26, 95% CI: 0.10 – 0.67, p = 0.006) and aCRT (HR 0.13, 95% CI 0.05 – 0.33, p < 0.0005) independently, compared to surgery only (Table S1). The addition of chemotherapy (aCRT) was also associated with improved OS compared to aRT in this group (HR: 0.46, 95%CI: 0.24–0.90, p = 0.022). Node stratification did not reveal any significant survival differences for the intermediate‐ and low‐risk patients.
4. DISCUSSION
Our findings suggest that intermediate‐risk patients may not benefit from the addition of AT to surgery (p = 0.380). Furthermore, the addition of chemotherapy may be associated with an increased risk of mortality compared to aRT (p = 0.046) and surgery alone (p = 0.050) in this group. This is in contrast to high‐risk patients, who show superior overall survival with AT (p = 0.013), which is further improved with the addition of chemotherapy to aRT (p = 0.021). This finding is most prominent among high‐risk patients with positive lymph nodes (p < 0.0005).
As supported in previous literature, aCRT confers a survival benefit for high‐risk patients, particularly in patients with node‐positive disease based on subset analysis. In contrast, our findings suggest that overtreatment in the adjuvant setting may be detrimental in patients without high‐risk based on pathologic features. The importance of accurately identifying patients that may benefit from adjuvant therapy is critical, given the potentially adverse impact on survival and treatment‐associated morbidity. Thus, adverse risk factors in surgical pathological outcomes should be closely scrutinized for risk stratification and treatment planning.
The lack of survival benefit from treatment intensification in the adjuvant setting for intermediate‐risk patients is supported by evidence from several studies in the current literature. In a large NCDB analysis, Osborn et al. found that the OS survival benefit did not persist for aCRT in patients without positive margins or ENE. 15 Another study found that aCRT was associated with an OS benefit for non‐oropharyngeal HNSCC patients with T1‐4 N2‐3 disease who were younger than 70 years of age, but not for those who were older than 70 years or had T3‐4 N0‐1 disease. 24 Among high‐risk patients in our study, we recapitulate the notion that aCRT (HR = .30) is beneficial, as has been consistently shown in other studies. Our study builds upon this prior evidence by demonstrating that nodal status appears to differentially affect treatment response in this group (Table S1). 11 , 12 , 13 , 14 , 25
A recent study published by Yan et al. used a similar methodology to investigate the effect of adjuvant chemoradiation therapy on OS for HPV‐negative HNSCC patients receiving primary surgical resection with negative margins from the National Cancer Database. 26 Patients classified as N2a with a single‐positive lymph node <3 cm and pathologic ENE did not have an OS benefit with CRT relative to adjuvant radiation alone (HR 0.98; 95% CI, 0.74–1.30). These findings were similar to our intermediate‐risk cohort. Despite the presence of pathologic ENE, the overall risk may have been mitigated by the presence of only a single‐positive node and negative surgical margins in this cohort. These findings suggest that even more nuanced pathologic risk scoring systems may be warranted to optimize treatment selection and provide individualized cancer care for patients. This remains a promising area for future research.
The harms of overtreatment in cancer care are often overlooked, and they warrant more discussion. The cytotoxic properties of cisplatin chemotherapy combined with radiation is associated with significant treatment‐related morbidity and mortality, especially in older patients. 27 , 28 Acute and chronic effects of combined therapy include mucositis, dysphagia, chronic pain, salivary gland dysfunction, infection, neutropenia, tracheostomy tube dependence, and feeding tube dependence. 27 , 28 , 29 Patients should understand that aCRT in the intermediate‐ or low‐risk setting may diminish functionality and quality of life with potentially little survival benefit, as found in our analysis.
Another main finding of our study is the low percentage of adherence to National Comprehensive Cancer Network (NCCN) guidelines. Patients were enrolled in the CHANCE study between 2002 and 2006. Data from EORTC 22391 and RTOG 95–01 were published in 2004, and it can take years before treatment guidelines are fully integrated into clinical practice. While treatment decisions in this study may not reflect current practice in 2020, investigators who hope to improve survival outcomes via treatment intensification should proceed with caution. This is perhaps most relevant in intermediate‐risk disease, which spans a broad range of clinical and surgical factors. For example, a patient with pathologic T3 disease should only receive adjuvant radiation, whereas a patient with T4 disease, LVSI, and PNI should be considered for aCRT. Unfortunately, our study did not analyze survival outcomes based on a cumulative number of risk factors. Adherence to treatment guidelines remains critical, 30 as many HNSCC studies report anywhere from 17% to 50% treatment non‐compliance. 30 , 31 , 32 The NCCN guidelines’ category 2A recommendation for aRT (uniform NCCN consensus) in high‐risk patients dates back to the early 2000s. 7 , 33 Nevertheless, 24% of high‐risk patients in our study received no AT.
Our study has several limitations. The primary limitation is the retrospective nature of our analysis. As discussed, primary data collection ended in 2006, so treatment patterns may not reflect current practice guidelines. Additionally, pathologic examination of lymph node status (ENE evaluation) was substantially different compared to current practice. Standard evaluation was limited to one histologic section of each node, whereas standardized reporting and College of American Pathologist protocols were not available. Historically, ENE was used to describe a grossly positive or matted node (macroscopic); however, currently, ENE can include a 1 mm focus extending from an equally small metastatic focus (microscopic). Another important limitation is the use of AJCC 7th Edition guidelines, which were available at the time of data collection. To help account for this, we stratified our multivariable analyses by pathological risk category to adjust for tumor characteristics captured in updated staging guidelines. Also, some of the post hoc subgroup analyses were limited by small sample sizes. Another limitation is that HPV‐positive oropharyngeal cases were excluded based on p16‐status alone. While p16 is considered a reliable marker for HPV infection, there is potential for a small number of p16‐positive HPV‐negative cases as shown in other studies. 34 Finally, the full records for adjuvant therapy received were incomplete. The majority of patients treated with aCRT in this analysis received cisplatin; however, cumulative dosing, and administration schedules (bolus vs. weekly) were not available. Similarly, patients were scheduled to receive the standard of care six weeks of aRT, however, the choice of RT used (conventional vs. intensity modulated) and cumulative dose received was not recorded.
The strengths of our study include a large population‐based sample with complete information on surgical pathologic outcomes. Additionally, our analysis adjusted for potential confounders such as age, sex, race, smoking history, alcohol use, and TNM stage. Given the large sample size and adjustment set, we believe our OS estimates represent true differences in survival by treatment modality and pathologic risk category. These findings help fill an important gap in the current literature and identify areas for future research. Specifically, novel combinatorial therapies such as concurrent radiation therapy plus immunotherapy should be evaluated to improve overall survival outcomes in intermediate‐risk, HPV‐negative patients.
5. CONCLUSION
While the use of aRT and aCRT appears to be associated with improved overall survival in high‐risk HPV‐negative HNSCC patients, the same benefit may not be observed in patients with intermediate‐risk disease. In high‐risk patients, node status may be an important parameter of response to treatment. Optimization of risk stratification has the potential to improve treatment selection and survival outcomes for patients with HPV‐negative HNSCC. Additionally, the integration of patient‐reported quality of life measures per treatment modality and risk category may inform decision‐making.
ETHICAL APPROVAL
The study was approved by the Institutional Review Board (IRB) of the University of North Carolina at Chapel Hill (Approval ID: 17–1220) and all participating institutions.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Conceptualization: SS, DF; Data Curation: JT, NL, DF; Formal Analysis: JT, NL, DF; Funding Acquisition: AO; Investigation: JT, NL, DF, SS; Methodology: JT, NL, DF, SS; Project Administration: SS; Resources: AO, JW, SS; Software: JT, NL, DF; Supervision: SS; Validation: JT, NL; Visualization: JT, NL; Writing ‐ Original Draft: JT, NL, SS; Writing ‐ Review and Editing: All authors.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was funded by the following Grants: National Cancer Institute, Award Number: R01‐CA90731. The authors thank Dr. Antonio L. Amelio at the University of North Carolina at Chapel Hill for Hill for helpful suggestions during the preparation of this manuscript.
J. Tasoulas and N. Lenze are co 1st authors.
Contributor Information
Jason Tasoulas, Email: siddharth.sheth@med.unc.edu.
Nicholas R. Lenze, Email: siddharth.sheth@med.unc.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Alsahafi E, Begg K, Amelio I, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10(8):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El‐Naggar AKCJ, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th Edition ed. vol 9. WHO Classification of Tumours. France: IARC Press; 2016. [Google Scholar]
- 3. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271‐289. 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4. Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16(11):669‐683. 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 5. Giraldi L, Leoncini E, Pastorino R, et al. Alcohol and cigarette consumption predict mortality in patients with head and neck cancer: a pooled analysis within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann Oncol. 2017;28(11):2843‐2851. 10.1093/annonc/mdx486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitd LB, Scanlon CS, D'Silva NJ. Perineural invasion in head and neck cancer. J Dent Res. 2018;97(7):742‐750. 10.1177/0022034518756297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NCCN . Head and neck cancers, version 1.2019. Updated May 3. 2019; https://www.ncbi.nlm.nih.gov/pubmed/23946171.
- 8. Mannelli G, Comini LV, Piazza C. Surgical margins in oral squamous cell cancer: intraoperative evaluation and prognostic impact. Curr Opin Otolaryngol Head Neck Surg. 2019;27(2):98‐103. 10.1097/MOO.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 9. Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: a systematic review and meta‐analysis. Oral Oncol. 2016;62:60‐71. 10.1016/j.oraloncology.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 10. Wurdemann N, Wagner S, Sharma SJ, et al. Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol. 2017;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945‐1952. 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 12. Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937‐1944. 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 13. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843‐850. 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 14. Cooper JS, Zhang Q, Pajak TF, et al. Long‐term follow‐up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high‐risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198‐1205. 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osborn VW, Givi B, Rineer J, et al. Patterns of care and outcomes of adjuvant therapy for high‐risk head and neck cancer after surgery. Head Neck. 2018;40(6):1254‐1262. 10.1002/hed.25103. [DOI] [PubMed] [Google Scholar]
- 16. Zumsteg ZS, Luu M, Kim S, et al. Quantitative lymph node burden as a ‘very‐high‐risk’ factor identifying head and neck cancer patients benefiting from postoperative chemoradiation. Ann Oncol. 2019;30(1):76‐84. 10.1093/annonc/mdy490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ringash J, Fisher R, Peters L, et al. Effect of p16 status on the quality‐of‐life experience during chemoradiation for locally advanced oropharyngeal cancer: a substudy of randomized Trial Trans‐Tasman Radiation Oncology Group (TROG) 02.02 (HeadSTART). Int J Radiat Oncol Biol Phys. 2017;97(4):678‐686. 10.1016/j.ijrobp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 18. Hoxbroe Michaelsen S, Gronhoj C, Hoxbroe Michaelsen J, Friborg J, von Buchwald C. Quality of life in survivors of oropharyngeal cancer: a systematic review and meta‐analysis of 1366 patients. Eur J Cancer. 2017;78:91‐102. 10.1016/j.ejca.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 19. Chen W‐C, Lai C‐H, Fang C‐C, et al. Identification of high‐risk subgroups of patients with oral cavity cancer in need of postoperative adjuvant radiotherapy or chemo‐radiotherapy. Medicine (Baltimore). 2016;95(22):e3770. 10.1097/MD.0000000000003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krstevska V. Evolution of treatment and high‐risk features in resectable locally advanced Head and Neck squamous cell carcinoma with special reference to extracapsular extension of nodal disease. J BUON. 2015;20(4):943‐953. [PubMed] [Google Scholar]
- 21. Giacalone NJ, Qureshi MM, Mak KS, et al. Adjuvant chemoradiation does not improve survival in elderly patients with high‐risk resected head and neck cancer. Laryngoscope. 2018;128(4):831‐840. 10.1002/lary.26798. [DOI] [PubMed] [Google Scholar]
- 22. Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21(4):567‐575. 10.1007/s10552-009-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazul AL, Taylor JM, Divaris K, et al. Oral health and human papillomavirus‐associated head and neck squamous cell carcinoma. Cancer. 2017;123(1):71‐80. 10.1002/cncr.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen MM, Colevas AD, Megwalu U, Divi V. Survival benefit of post‐operative chemotherapy for intermediate‐risk advanced stage head and neck cancer differs with patient age. Oral Oncol. 2018;84:71‐75. 10.1016/j.oraloncology.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 25. Lin SS, Massa ST, Varvares MA. Improved overall survival and mortality in head and neck cancer with adjuvant concurrent chemoradiotherapy in national databases. Head Neck. 2016;38(2):208‐215. 10.1002/hed.23869. [DOI] [PubMed] [Google Scholar]
- 26. Yan F, Li H, Kaczmar JM, et al. Evaluating adjuvant therapy with chemoradiation vs radiation alone for patients with HPV‐negative N2a head and neck cancer. JAMA Otolaryngol–Head & Neck Surg. 2020;146(12):1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daly ME, Lau DH, Farwell DG, Luu Q, Donald PJ, Chen AM. Feasibility and toxicity of concurrent chemoradiation for elderly patients with head and neck cancer. Am J Otolaryngol. 2013;34(6):631‐635. 10.1016/j.amjoto.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 28. Rütten H, Pop LAM, Janssens GORJ, et al. Long‐term outcome and morbidity after treatment with accelerated radiotherapy and weekly cisplatin for locally advanced head‐and‐neck cancer: results of a multidisciplinary late morbidity clinic. Int J Radiat Oncol Biol Phys. 2011;81(4):923‐929. 10.1016/j.ijrobp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 29. Jamal N, Ebersole B, Erman A, Chhetri D. Maximizing functional outcomes in head and neck cancer survivors: assessment and rehabilitation. Otolaryngol Clin North Am. 2017;50(4):837‐852. 10.1016/j.otc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 30. Dronkers EA, Mes SW, Wieringa MH, van der Schroeff MP, Baatenburg de Jong RJ. Noncompliance to guidelines in head and neck cancer treatment; associated factors for both patient and physician. BMC Cancer. 2015;15(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamaker ME, Smorenburg CH, Bun RJ, et al. Age‐related differences in guideline adherence for head and neck cancer. J Geriatric Oncol. 2012;3(4):329‐336. 10.1016/j.jgo.2012.07.004. [DOI] [Google Scholar]
- 32. Roden D, Daniels K, Metkus J, et al. Evaluation of oncologic outcomes in head and neck cancer patients >/=80 years old based on adherence to NCCN guideline for postoperative adjuvant treatment. Head Neck. 2019;41(12):4128‐4135. 10.1002/hed.25950. [DOI] [PubMed] [Google Scholar]
- 33. NCCN . Head and neck cancers, version 1.2002. Updated May 3. 2019; https://www.ncbi.nlm.nih.gov/pubmed/23946171.
- 34. Rietbergen MM, Brakenhoff RH, Bloemena E, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De‐escalation trials. Ann Oncol. 2013;24(11):2740‐2745. 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


