Figure 3.
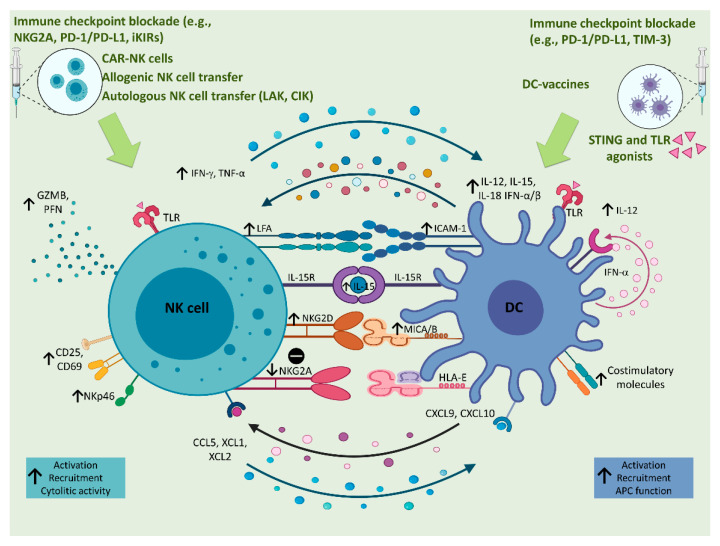
Implications of NK–DC crosstalk in liver cancer immunotherapy. Several immunotherapeutic approaches based on the administration of CAR-NK cells, allogenic or autologous NK cells or DC-vaccines are used in cancer patients to activate innate immune responses against tumor cells. The NK cell activation could also be reached through the administration of TLR agonists, like IQ and GDQ, which are, respectively, TLR7 and TLR7/8 agonists. NK cell activation on one hand leads to DC maturation, and on the other hand it is enhanced by the interaction with DCs, which in turn are activated by TLR agonists or STING. This bilateral activation involves the upregulation of CD69, CD25, NKp46, NKG2D, and the release of lytic enzymes (e.g., GZMB and PFN) by NK cells. On the other hand, activated DCs undergo upregulation of costimulatory molecules, NKG2D ligands (e.g., MICA/B) and an increased release of IFN-α, which in a positive loop, also contributes to the production of IL-12 that activates NK cells to produce high levels of IFN-γ and TNF-α. In addition, IL-15 and IL-18 sustain NK cell survival and activation that are also favored by the direct contact between NK cells and DCs through the interaction between NKG2D and its ligands, and LFA and ICAM-1, on NK cell and DC surface, respectively. Moreover, the reciprocal recruitment of both cellular populations is supported by the production of CXCL9 and CXCL10 by DCs and CCL5, XCL1, and XCL2 by NK cells. Other immunotherapeutic approaches, aimed at blocking inhibitory receptors, like the NKG2A, iKIRs, and the PD-1/PD-L1 axis, favor the activation of NK cells, consequently allowing DC maturation and activation, which could be also enhanced by the administration of immune checkpoint inhibitors against PD-1/PD-L1 or TIM-3. [CAR-NK cells, Chimeric Antigen Receptor natural killer cells; CCL5, C-C Motif Chemokine Ligand 5; CXCL10, Chemokine (C-X-C motif) ligand 10; CXCL9, Chemokine (C-X-C motif) ligand 9; DCs, dendritic cells; GDQ, Gardiquimod; GZMB, granzyme B; HLA-E, Major Histocompatibility Complex, Class I, E; IFN-α, interferon α; IFN-β, interferon β; IFN-γ, interferon γ; IL-12, interleukin 12; IL-15, interleukin 15; IL-15R, interleukin 15 receptor; IL-18, interleukin 18; iKIRs, inhibitory Killer-cell immunoglobulin-like receptors; MICA/B, MHC class-I-related protein A/B; IQ, Imiquimod; NK cells, natural killer cells; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFN, perforin; STING, stimulator of interferon genes; TIM-3, T-cell immunoglobulin domain and mucin domain 3; TLR, toll-like receptor; TNF-α, tumor necrosis factor α; XCL1, Chemokine (C motif) ligand 1; XCL2, Chemokine (C motif) ligand 2; and XCR1, X-C Motif Chemokine Receptor 1]. Created with BioRender.com (accessed on 16 March 2021).