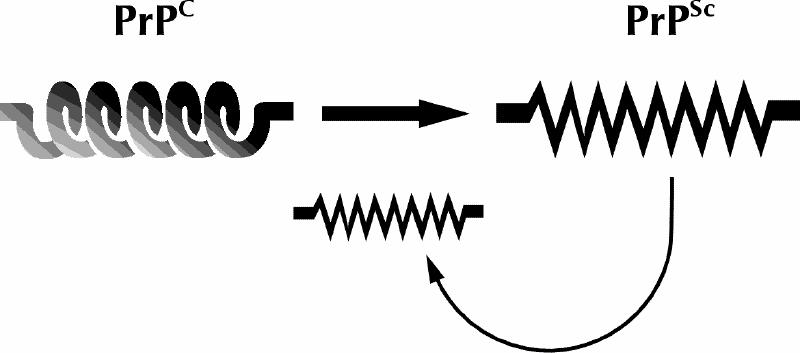
Fig. 1: Schematic depiction of the prion hypothesis. A normally folded cellular form of the prion protein called PrPCC, which consists largely of α helix, for unknown and perhaps diverse reasons adopts an alternative conformation (PrPSc) that is rich in β-sheet structure, has a tendency to aggregate and possesses additional properties that lead directly or indirectly to CNS toxicity. Once formed, molecules in the misfolded conformation have a tendency to stimulate the formation of additional PrPSc by post-translational recruitment of PrPC.
