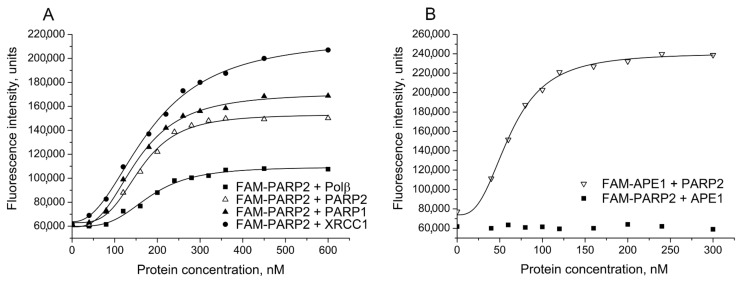
Figure 1.
Detection of PARP2 complexes with various BER proteins by fluorescence titration of FAM-PARP2 or FAM-APE1. The FAM-labelled protein (40 nM) was excited at 482 nm in the absence or presence of increasing concentrations of the binding partner indicated, and the relative fluorescence intensities were monitored at 530 nm. Typical titration curves representing binding of FAM-PARP2 to Polβ, PARP2, PARP1, and XRCC1 (A), and complex formation between FAM-APE1 and PARP2 (B) show the best fits of the four-parameter equation (with R2 values exceeding 0.98); no detectable change in the fluorescence intensity of FAM-PARP2 in the presence of APE1 is demonstrated (B).