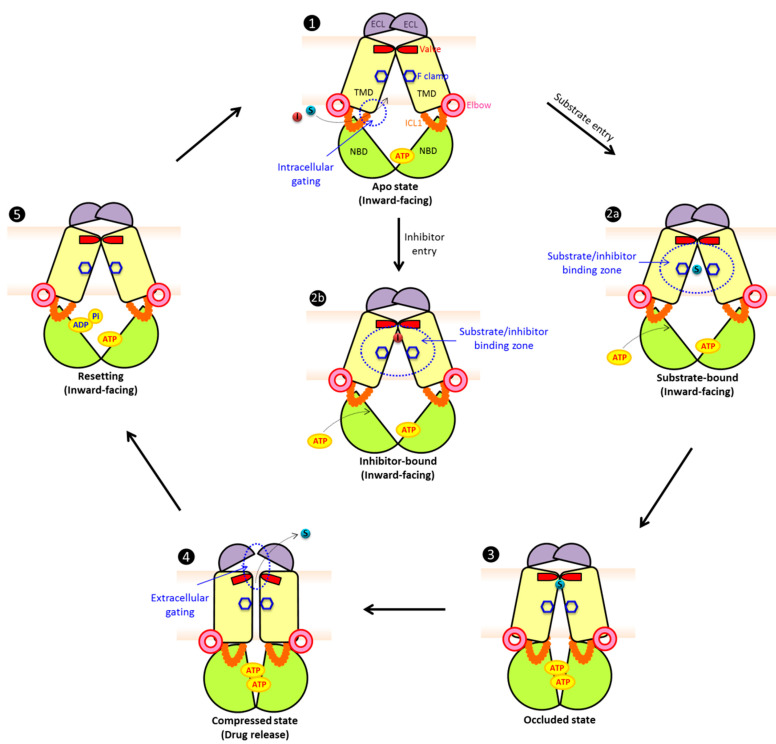
Figure 4.
Proposed catalytic cycles of mammalian/fungal type II exporters (ABCG/PDR). In the apo substrate-free state (1), the exporter adopts an inward-facing conformation. We propose that NBDs are open with ATP present in at least one NBD or both, which mediates partial NBD dimerization leaving only one accessible ATP-binding region. The intracellular gate(s) at the transmission interface provides access for substrate or inhibitor entry. The aromatic rings at the conserved F clamp form accessible binding sites at the closed transporter valve subtending the closed ECL. Drug substrates (2a) or inhibitors (2b) can enter through intracellular gate(s), preceding their trapping in distinct binding zones in the central cavity. Binding of ATP at the second binding site or both triggers full NBD dimerization and triggers a first conformational change, setting an occluded state (3). The communication between NBD and TMD is regulated via a rigid triple helical bundle (THB) as a key part of the transmission interface. The NBD dimerization compresses the central cavity space to drive substrate movement through the translocation, thus engaging a push and squeeze motion to open the valve. Substrates shift into the upper cavity and are released by the subsequent opening of the ECL lid (4). ATP hydrolysis at one NBD site may be enough to reset the catalytic cycle and to convert the transporter molecule into the inward-facing drug-recognizing state (5). The structures show NBDs (green), elbow helix (pink), TMDs (yellow), ICL (orange), ECL (purple), phenylalanine clamp (blue hexagon), valve (red), substrates (cyan) and inhibitors (red). For more details and references see the main text.