Abstract
The mechanism of nigral dopaminergic neuronal degeneration in Parkinson’s disease (PD) is unknown. One of the pathological characteristics of the disease is the deposition of α-synuclein (α-syn) that occurs in the brain from both familial and sporadic PD patients. This paper constitutes a narrative review that takes advantage of information related to genes (SNCA, LRRK2, GBA, UCHL1, VPS35, PRKN, PINK1, ATP13A2, PLA2G6, DNAJC6, SYNJ1, DJ-1/PARK7 and FBXO7) involved in familial cases of Parkinson’s disease (PD) to explore their usefulness in deciphering the origin of dopaminergic denervation in many types of PD. Direct or functional interactions between genes or gene products are evaluated using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database. The rationale is to propose a map of the interactions between SNCA, the gene encoding for α-syn that aggregates in PD, and other genes, the mutations of which lead to early-onset PD. The map contrasts with the findings obtained using animal models that are the knockout of one of those genes or that express the mutated human gene. From combining in silico data from STRING-based assays with in vitro and in vivo data in transgenic animals, two likely mechanisms appeared: (i) the processing of native α-syn is altered due to the mutation of genes involved in vesicular trafficking and protein processing, or (ii) α-syn mutants alter the mechanisms necessary for the correct vesicular trafficking and protein processing. Mitochondria are a common denominator since both mechanisms require extra energy production, and the energy for the survival of neurons is obtained mainly from the complete oxidation of glucose. Dopamine itself can result in an additional burden to the mitochondria of dopaminergic neurons because its handling produces free radicals. Drugs acting on G protein-coupled receptors (GPCRs) in the mitochondria of neurons may hopefully end up targeting those receptors to reduce oxidative burden and increase mitochondrial performance. In summary, the analysis of the data of genes related to familial PD provides relevant information on the etiology of sporadic cases and might suggest new therapeutic approaches.
Keywords: mitochondria, vesicular transport, mitophagy, Lewy bodies, synuclein aggregation, familial Parkinson’s disease, early-onset Parkinson’s disease
1. Introduction
Parkinson’s disease is a prevalent neurodegenerative disease the first clinical symptoms of which include tremors and motor dysfunction. The number of cases with unknown causes is much higher than the number of familial cases; that is, cases due to known genetic alterations. Years ago, the percentage of cases of inheritable PD was estimated to be around 5%. Recent estimates increased this percentage because of Genetic-oriented research, which led to the discovery of more genes associated with the disease. In a recent multicenter study in a cohort of 1587 cases, mutations were found in 14.1% of patients [1] (see also [2,3]). Although familial cases often display early-onset symptoms, the main risk factor is age with 60 years being considered as the threshold for developing symptoms in sporadic cases. In these cases, many positive (e.g., caffeine) and negative (e.g., toxin exposure) risk factors are known [4,5,6,7,8,9,10,11,12,13], but the exact causes of why dopamine neurons of the substantia nigra die in both familial and sporadic cases remain unknown.
The work of Hornykiewicz and colleagues was instrumental for early therapeutic intervention in PD cases. They discovered the lack of dopamine in certain brain areas as the cause of motor symptoms and noticed that dopamine supplementation was not effective because the compound is unable to cross the blood–brain barrier. They discovered that L-DOPA (levodopa), the precursor of the neurotransmitter, was able to enter the brain and there be converted into dopamine. L-DOPA is still used in PD therapy. The need for chronic treatment and fluctuations in drug levels in the brain may lead to some side effects, mainly dyskinesia [14,15,16,17,18,19]. Side effects may be addressed via surgical procedures. Since the nineties, the technique has been refined and used with success by implanting electrodes that achieve what is known as deep-brain stimulation (DBS) [20,21,22,23,24,25,26,27,28]. Unfortunately, there is no therapeutic intervention that delays disease progression, that is, the neurodegeneration of dopaminergic nigral neurons.
PD results from death of dopamine-producing neurons of the substantia nigra. An imbalance of dopaminergic neurotransmission in this area of the brain leads to motor deficits, which first appear as tremors and difficulty starting to walk and moving limbs precisely. Be it cause or consequence, there is a main characteristic of pathological PD: the appearance of Lewy bodies formed by aggregates of a protein, α-synuclein (α-syn), the function of which has not yet been fully elucidated. PD has analogies and differences with other diseases known as α-synucleinopathies; the two other main α-synucleinopathies are dementia with Lewy bodies and multiple system atrophy (see [29,30,31]). The deposition of α-syn aggregates occurs in the brain of patients with both familial and sporadic PD. Finally, it should be noted that a theory has arisen that postulates that the risk of PD may be increased by some viral infections [32].
Taking into account the familial cases, i.e., mutations in SNCA, LRRK2, GBA, UCHL1, VPS35, PRKN, PINK1, ATP13A2, PLA2G6, DNAJC6, SYNJ1, DJ-1/PARK7 and FBXO7 genes, it appears that (i) mutant forms of α-syn can aggregate and lead to early-onset PD and (ii) that mutations in other genes lead to deposition of non-mutated α-syn and early onset of the EP. The present narrative review aims to propose a scenario in which these players are connected; that is, we would like to obtain information from familial cases that could help decipher the causes of dopaminergic denervation in all types of PD.
The approach is sustained in two qualitatively different datasets. First, a blind approach is used to find the connections between genes that have alterations associated with the disease. First, we selected genes related to PD familial cases (listed in Table 1) to find interactions among them (in a blind-like approach) using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING). Second, information from transgenic models was analyzed in the search for mechanisms of phenotypic alterations (focused on the CNS and, whenever possible, considering alterations in the substantia nigra/striatum). From these two independent sets of data we finally presented a picture of the potential events that occur in degenerating dopaminergic neurons.
Table 1.
Genes considered in this review and having mutations likely related to early-onset PD. See description of gene products in Table 2.
| Gene | Inheritance | Proposed Disease Mechanism | Disease Onset | Mutation | Frequency |
Confidence as
Actual PD Gene |
Year of
Discovery |
Animal Model | |
|---|---|---|---|---|---|---|---|---|---|
| Early | Late | ||||||||
| SNCA | D | GoF or overexpression | Often with dementia |
Missense or multiplication |
Very rare | Very high | 1997,2003 | + | |
| LRRK2 | D | GoF | X | Missense | common | Very high | 2004 | + | |
| GBA | D | Likely LoF | X | Missense or LoF | common | Very high | 2009 | + | |
| UCHL1 | D | LoF | NA | Missense | unclear | Low | 1998 | + | |
| VPS35 | D | LoF | X | Missense | Very rare | Very high | 2011 | + | |
| PRKN | R | LoF | X | Missense or LoF | rare | Very high | 1998 | + | |
| PINK1 | R | LoF | X | Missense or loss of function |
rare | Very high | 2004 | + | |
| ATP13A2 | R | LoF | Atypical PD | Missense or LoF | Very rare | Very high | 2006 | + | |
| PLA2G6 | R | LoF | X | Missense or LoF | rare | Very high | 2009 | NA | |
| DNAJC6 | R | LoF | X | Missense o LoF | Very rare | High | 2012 | NA | |
| SYNJ1 | R | LoF | (often) Atypical PD | Missense or loss of function |
Very rare | Very high | 2013 | NA | |
| DJ-1/PARK7 | R | LoF | X | Missense | Very rare | Very High | 2003 | + | |
| FBXO7 | R | LoF | X | Missense | Very rare | Very High | 2008 | NA | |
D: Dominant; R: Recessive; NA: Not available; GoF: Gain-of-function; LoF: Loss-of-function.
2. STRING Analysis Restricted to Connections Obtained from Experimental, Gene Fusion, Protein Analogy or Proximity Data
STRING 11.0 version was used in the analysis of the potential connections among genes in Table 1. Both software and platform are incorporated into the European “ELIXIR Core Data Resources”; it is freely available and analyses were performed on-line in https://string-db.org (accessed on 4 March 2021).
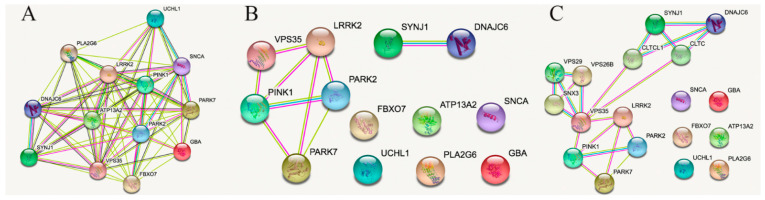
The STRING default setting for the genes associated with familial PD and listed in Table 1 leads to Figure 1A, in which virtually all genes (or gene products) are interconnected. We have omitted genes that were more recently associated with familial PD but for which less data was available [33,34]. Figure 1A shows that PLA2G6 is linked to UCHL1 and BNAJc6 only by neighborhood, meaning that there are no studies confirming whether or not there is any functional link between them. Obviously, at the bibliographic level, all genes are related to Parkinson’s disease; therefore, we next eliminated the connections derived from the mention of genes in papers related to PD.
Figure 1.
STRING analysis of connections between (human) genes in Table 1. Panel (A): using by default settings. Panel (B). Restricted settings (mainly leaving aside connections driven by PD-related literature; see text for details). Panel (C): Interaction-enriched connection pattern (see text for details). Line color code: sky blue, known interactions from curated databases; magenta, experimentally determined interactions; green, predicted from neighborhood; red, predicted from gene fusions; blue, predicted from gene co-occurrence; pastel green, textmining; black, coexpression; and clear violet, protein homology.
At first glance, avoiding the bias due to appearance in PD-related papers, the number of interactions is markedly reduced. On the one hand, restricting connections to those derived from experimental data, gene fusion, protein analogy, or proximity, 5 gene products were found to be interconnected (See Figure 1B). All except the PARK2/PARK7 potential interaction were (according to STRING database) experimentally determined (pink line between elements). On the other hand, an interaction between SYNJ1 and DNAJC6 products was identified. Overall, 6 elements were not connected to each other. If we enrich the pattern, including partners that were not present in the initial analysis, we obtain Figure 1C, in which the 6 elements still remain non-connected. Patterns arising using these 6 genes as individual elements are in the Supplementary Material Figure S1 (settings exclude co-appearance in PD-related papers). Whereas 5 of them showed interrelationships with other proteins, the product of PLA2G6, 85/88 kDa calcium-independent phospholipase A2, did not display any interaction related to experiments, gene fusion, co-expression, co-occurrence or neighborhood, meaning that its role is not yet well deciphered. In contrast, the protein encoded by UCLH1, ubiquitin carboxyl-terminal hydrolase isozyme L1, is an element with relevant links that include experimental data, protein homology and gene and protein coexpression. Actually, the search of mechanisms for non-gene-associated PD cases is the aim of this paper.
3. Lessons from Interactions among PARK2, PARK7, PINK1, LRRK2 and VPS35
The proteins encoded by PARK2, PARK7, PINK1, LRRK2 and VPS35 have a complementary role related to subcellular organelles, mainly the lysosome. Furthermore, practically all gene products participate in protein processing, in which ubiquitination is a relevant mechanism. Ubiquitination was described decades ago as a mechanism for targeting a protein for degradation [35,36,37]. If the processes involving ubiquitination are not completely balanced, the handling of proteins is inadequate, and the cell is damaged. Apart from ubiquitin-protein ligase (PARK2/parkin), other elements also participate in ubiquitination-mediated processes that lead to proteasomal degradation. This view is in agreement with the finding that some inherited PD cases are associated with the PARK7, PARK6, and PARK2 loci [38]. Mutant forms of DJ-1/PARK7 that are associated with PD lead to differential interactions with E3 ubiquitin-protein ligase leading to altering protein processing or leading to oxidative stress [39]. PINK1 is genetically associated with parkin. The discovery first made in Drosophila melanogaster [40,41] was later confirmed in humans [42]. In 2005, Smith and colleagues demonstrated an interaction of leucin-rich repeat kinase 2 (product of LRRK2) with parkin and showed that mutant forms of the kinase may induce neuronal death [43]. Vacuolar protein sorting-associated protein 35 is involved in the vesicular transport of vesicles from mitochondria. Such mitochondrial-derived vesicles are linked to ubiquitination in a complex way (see [44] for details). Furthermore, the ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL1) and the F-box component of protein 3 ubiquitin-protein ligase complex (FBXO7), have a role in targeting protein degradation using the ubiquitination pathway.
4. Lessons from Interactions between and from SYNJ1 and DNAJC6
The link between these two genes is quite straightforward. On the one hand, SYNJ1 codes for Synaptojanin-1, which is a quite unspecific phosphatase because it may degrade different phosphoinositides, which are key components on biological membranes. On the other hand, DNAJC6 codes for auxilin, which is a protein phosphatase involved in vesicle handling; in addition, auxilin belongs to a family of chaperones (DNAJ/HSP40). Knowledge of the function of these proteins is only partial, but at present they seem related to early events occurring shortly after endocytosis. Data on their involvement in the trafficking of intracellular vesicles or targeting of proteins for degradation is scant. In fact, genetic deletion of DNAJC6 in mice results in death due to the inability to perform clathrin-mediated endocytosis [45]. A study in Saccharomyces cerevisiae confirms that both the product of Dnajc6 and Synaptojanin-1 are necessary for the uncoating of clathrin, the proper trafficking of endosomes and the delivery of endocytic cargo [46,47].
5. Enriching the Network
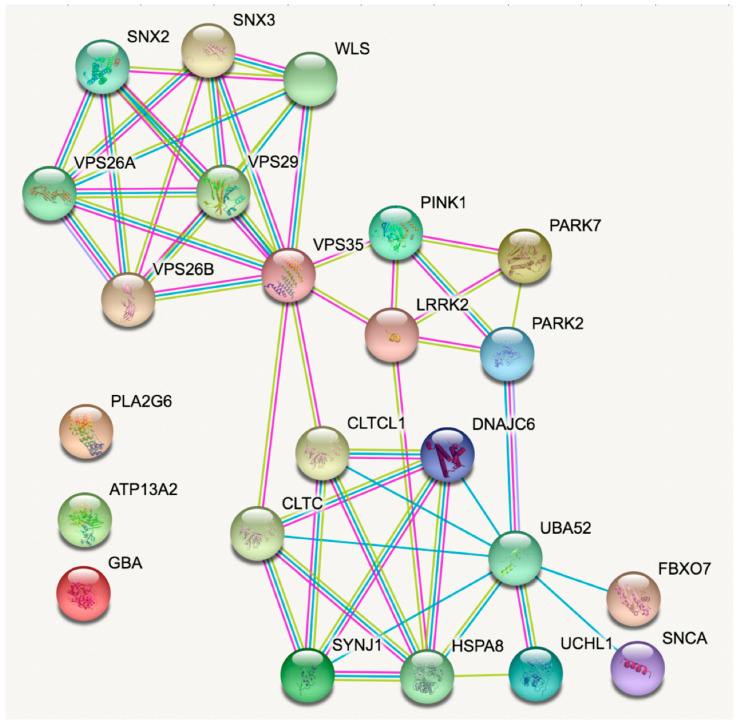
STRING is a powerful tool as it permits the enrichment of networks but avoiding the bias of parkinsonism in the enrichment. In doing so, the PARK2/PARK7/PINK1/LRRK2/VPS35 network connects with the SYNJ1/DNAJC6 network. Obviously, this only is possible if more genes, related or not with PD, are considered. The resulting enriched network is in Figure 2.
Figure 2.
Enriched STRING analysis of connections between (human) genes in Table 1. Line color code; sky blue, known interactions from curated databases; magenta, experimentally determined interactions; green, predicted from neighborhood; red, predicted from gene fusions; blue, predicted from gene co-occurrence; pastel green, textmining; black, coexpression; and clear violet, protein homology.
First of all, Figure 2 shows that only 3 PD-related genes are not connected to the rest (GBA, ATP132A and PLA2G6). Importantly, VPS35 becomes a relevant node that is connected, on the one side to several proteins involved in intracellular vesicular trafficking (vesicular sorting proteins, sorting nexins and Wnt Ligand Secretion Mediator, WLS) and, on the other side, directly to two gene products related to PD (PINK1 and LRRK2) and, indirectly with all other PD-related genes except GBA, ATP132A and PLA2G6. Also of note is that the network involving most of the PD-related genes (Figure 2, see connections involving VPS35) contains elements having a variety of functions in common: ubiquitination, clathrin-mediated endocytosis and phosphoinositide handling. In the far bottom right of Figure 2, α-synuclein (α-syn) appears only connected to ubiquitin-60S ribosomal protein L40 due to protein homology.
In summary, the study of genes related with inherited forms of PD shows that the products of the genes participate in intracellular vesicular transport and in clathrin-mediated endocytosis, which is subsequent to the activation of cell surface G protein-coupled receptors (GPCRs).
6. Lessons from Transgenic Animals
Transgenic animals have been fundamental to understanding disease mechanisms. While in non-neurological diseases many models are based on the genetic inactivation of a gene in neurodegenerative diseases (providing so-called knockout (KO) animals), the approach of overexpressing a mutated or non-mutated form of a human protein is often used. In a set of tables, we present a summary of the data obtained from transgenic animals, both generated for the purpose of better understanding the disease of PD, or previously generated for another purpose, but having provided data on alterations of the functionality of the nervous system.
Data from transgenic animals of other PD-related genes and the relevant references are included in Table 3 and Table 4 and in Supplementary Table S1. After a selection of ad hoc papers, these 3 tables were constructed to summarize data from a significant number of studies indicating for each the transgenic model and the main anomalies found after in vivo or in vitro studies.
Table 3.
Findings in animal models related to Snca.
| Animal Model (s) | In Vivo | In Vitro | Expression Level | Main Findings | Ref. |
|---|---|---|---|---|---|
| Mice harbouring Sncatm1Nbm mutation (n.s.n) | X | Snca KO mice | Modulates microglial activation phenotype Increased levels of reactive marker proteins |
[49] | |
| Mice harbouring Sncatm1Nbm mutation (n.s.n) | X | Snca KO mice | Altered palmitate metabolism | [50] | |
| Mice harbouring Sncatm1Nbm mutation (n.s.n) | X | Snca KO mice | Mitochondrial lipid abnormality Electron transport chain impairment |
[51] | |
| Mice harbouring Sncatm1Nbm mutation (n.s.n) | X | X | Snca KO mice | Synaptic vesicle depletion | [52] |
| C57BL/6N-Sncatm1Mjff/J | X | X | Snca KO mice | Non-altered mitochondrial bioenergetics | [53] |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | α-syn restricts RNA viral infections in the brain | [54] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | ROS and NOS-2 decreased in mature erythrocytes |
[55] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | No modification in pale body-like inclusion Altered proteasome function |
[56] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | Inhibition of intrasynaptic vesicle mobility Maintains recycling-pool homeostasis |
[57] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | Cognitive impairments | [58] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | Vulnerability of peripheral catecholaminergic neurons to MPTP not regulated by α-synuclein | [59] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | Snca KO mice | Resistant to mitochondrial toxins | [60] | |
| B6; 129 × 1-Sncatm1Rosl/J | X | X | Snca KO mice | Deficits in the nigrostriatal dopamine system | [61] |
| B6; 129 × 1-Sncatm1Rosl/J C57Bl/6JOlaHsd |
X | X | Snca KO mice | Decreased impulsivity | [62] |
| B6; 129 × 1-Sncatm1Rosl/J C57Bl/6JOlaHsd |
X | Snca KO mice | Neuromuscular pathology | [63] | |
| B6; 129 × 1-Sncatm1Rosl/J C57Bl/6JOlaHsd |
X | X | Snca KO mice | Decreased reuptake of dopamine in the dorsal striatum | [64] |
| B6; 129 × 1-Sncatm1Rosl/J (Snca KO) B6-TgHSNCGtm1VLB (Sncg KO) |
X | X | -α-syn (Snca) KO mice -ɣ-syn (Sncg) KO mice |
Altered dopamine metabolism | [65] |
| B6; 129 × 1-Sncatm1Rosl/J (Snca KO) B6-TgHSNCGtm1VLB (Sncg KO) |
X | X | -α-syn (Snca) KO mice -ɣ-syn (Sncg) KO mice -α-ɣ-syn double KO mice |
Increased striatal dopamine release Hyperdopaminergic signs |
[66] |
| Triple-synuclein-KO (TKO) | X | X | α-syn (Snca), ß-syn (Sncb) and ɣ-syn (Sncg) triple KO mice | Functional alterations to the nigrostriatal system | [67] |
| Triple-synuclein-KO (TKO) | X | X | α-syn (Snca), ß-syn (Sncb) and ɣ-syn (Sncg) triple KO mice | Altered synaptic vesicle endocytosis α -, ß -, or γ-synucleins are functionally redundant |
[68] |
| Triple-synuclein-KO (TKO) | X | X | α-syn (Snca), ß-syn (Sncb) and ɣ-syn (Sncg) triple KO mice | Age-dependent neuronal dysfunction | [69] |
| Triple-synuclein-KO (TKO) See paper for further details on model(s) |
X | X | α-syn (Snca), ß-syn (Sncb) and ɣ-syn (Sncg) triple KO mice. Mice overexpressing human SNCA mutants PARK1/hA30P or PARK4/hα-syn |
Effects on presynaptic architecture | [70] |
| B6;129 × 1-Sncatm1Rosl/J B6.CgTg (SNCA) OVX37Rwm Sncatm1Rosl/J |
X | X |
-Snca KO mice -SNCA overexpressing mice |
Blockade of TrkB neurotrophic effect | [71] |
| B6;DBA-Tg (Thy1-SNCA) 61Ema | X | SNCA overexpressing mice | Impairment of mitochondrial function Elevated ROS in brain mitochondria |
[72] | |
| B6;DBA-Tg (Thy1-SNCA) 61Ema | X | SNCA overexpressing mice | Alterations in corticostriatal synaptic plasticity | [73] | |
| B6;DBA-Tg (Thy1-SNCA) 61Ema | X | X | SNCA overexpressing mice | Alterations in calcium buffering capacity | [74] |
| (Thy1)-(WT)a-syn |
X | SNCA overexpressing mice | Enhanced axonal degeneration after peripheral nerve lesion |
[75] | |
| B6.Cg-Tg (SNCA) OVX37Rwm Sncatm1Rosl/J | X | X | SNCA overexpressing mice | Deficits in dopaminergic transmission precede neuronal loss | [76] |
| B6.Cg-Tg (SNCA) OVX37Rwm Sncatm1Rosl/J | X | SNCA overexpressing mice | Impairment of macroautophagy in dopaminergic neurons | [77] | |
| B6; 129 × 1-Sncatm1Rosl/J neurons transfected with SNCA or TsixK-SNCA | X | Cultured neurons from Snca KO mice overexpressing SNCA or TsixK-SNCA | α-syn multimers attenuate synaptic vesicle recycling | [78] | |
| S129 mutations performed on mice harbouring Sncatm1Nbm mutation (n.s.n.) | X | X | S129D-SNCA (phosphomimetic) or S129A- SNCA (non-phosphorylatable) overexpressing Snca KO mice | No abnormalities detected (in vivo) | [79] |
| FVB;129S6-Sncatm1Nbm Tg (SNCA*A53T) 1Nbm Tg (SNCA*A53T) 2Nbm/J FVB;129S6-Sncatm1Nbm Tg (SNCA*A30P) 1Nbm Tg (SNCA*A30P) 2Nbm/J |
X | X | A53T-SNCA or A30P-SNCA overexpressing Snca KO mice | Enteric nervous system abnormalities | [80] |
| M83KO mice resulting from crossing M83 line with B6; 129X1-Sncatm1Rosl/J line | X | A30P-SNCA overexpressing Snca KO mice | Dopaminergic neurodegeneration |
[81] | |
| B6.Cg-Sncatm1Rosl Tg (SNCA*A30P) 192Rwm/J | X | X | A30P-SNCA overexpressing Snca KO mice | Region-specific deficits in dopamine signaling | [82] |
| FVB;129-Tg (Prnp-SNCA*A53T) AAub/J | X | X | A53T-SNCA overexpressing mice | Dysfunctional neurotransmission Impaired synaptic plasticity |
[83] |
| FVB;129-Tg (Prnp-SNCA*A53T) AAub/J | X | X | A53T-SNCA overexpressing mice | Neuronal dysfunction in the absence of aggregate formation Behavioral alterations |
[84] |
| NTac:SD-Tg (SNCA*A53T) 268Cjli | X | X | A53T-SNCA overexpressing mice | Dynamic changes in striatal mGluR1 but not mGluR5 |
[85] |
| B6;C3-Tg (Prnp-SNCA*A53T) 83Vle/J (also known as:A53T α-synuclein transgenic line M83) |
X | X | Brain inoculation with brain homogenates from older Tg mice or with human α-syn fibrils in Tg A53T-SNCA overexpressing mice | Inoculation initiates a rapidly progressive neurodegenerative α-synucleinopathy | [86] |
| NTac:SD-Tg (SNCA*E46K) 70CJLi | X | X | E46K-SNCA overexpressing rats | Enhanced vulnerability to mitochondrial impairment |
[87] |
| Double transgenic Uchl1tm1Dgen:Thy1-maSN | X | X |
Uch-L1 KO + Snca overexpressing mice |
Excess α-syn worsens disease in mice lacking Uch-L1 | [88] |
| B6; 129×1-Sncatm1Rosl/J C57B/6jxSJL F3, Tg5093 |
X |
-Snca KO mice -A53T-SNCA overexpressing mice |
α-syn expression levels do not significantly affect proteasome function | [89] | |
| See paper for details of the animal model(s) | X | -A53T-SNCA transfected in WT neurons -Snca KO mice |
Altered fatty acid composition of dopaminergic neuron PUFAs enhance α-syn oligomerization |
[90] | |
| B6;129-Sncatm1Sud Sncbtm1.1Sud/J See also paper for further details |
X | -Cultured neurons from Snca KO mice overexpressing SNCA -See paper for other models used |
Inhibition of synaptic vesicle reclustering after endocytosis | [91] | |
| B6;129×1-Sncatm1Rosl/J | X | X | α-syn fibrils gut-injected in Snca KO mice | α-syn transneuronal propagation from the gut to the brain | [92] |
| WT vs. KOM2 | X | Glial cytoplasmic inclusions-α-syn or Lewy Bodies-α-syn injected in WT mice vs. mice that express human α-syn only in oligodendrocites (KOM2) | Cellular milieu affects pathology of α-syn | [93] |
n.s.n.: Non standard nomenclature.
Table 4.
Findings in animal models related to Prkn, Lrrk2 and Pink1.
| Gene | Animal Model (s) | In Vivo | In Vitro | Expression Level | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| PRKN | B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Independent regulation of parkin ubiquitination and alpha-synuclein clearance | [96] |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Accelerated microtubule aging in dopaminergic neurons | [97] | |
| B6.129S4-Prkntm1Shn/J |
X | Prkn KO mice | Myotubular atrophy Impaired mitochondrial function and smaller myofiber area |
[98] | ||
| B6.129S4-Prkntm1Shn/J | X | Prkn KO mice | Parkin mediates the ubiquitination of VPS35 Reduced WASH complexes |
[99] | ||
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | ER stress and induced inflammation levels | [100] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Behavioral impairments Amplified EtOH-induced dopaminergic neurodegeneration, oxidative stress and apoptosis Dysfunction of mitochondrial autophagy |
[101] | |
| B6.129S4-Prkntm1Shn/J |
X | Prkn KO mice | Parkin promotes proteasomal degradation of Synaptotagmin IV | [102] | ||
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | SNPH Cargo vesicle generation not affected | [103] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Exacerbated mitochondrial dysfunction in neurons | [104] | |
| B6.129S4-Prkntm1Shn/J |
X | X | Prkn KO mice | Increased sensitivity to myocardial infarction Reduced survival after the infarction Reduced mitophagy |
[105] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Parkin antagonizes the death potential of FAF1 | [106] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Acutely sensitivity to oxidative stress Inability to maintain Mcl-1 levels Death of dopaminergic neurons |
[107,108] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Aberrant behavioral response to dopamine replacement therapy in PD | [109] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Resisted weight gain, steatohepatitis, and insulin resistance Abolished hepatic fat uptake |
[110] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Reductions in the total catecholamine release Impaired LTP and LTD Normal levels of dopamine receptors and dopamine transporters |
[111] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Requiring inflammatory stimulus for nigral DA neuron loss | [112] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Reduced respiratory capacity mitochondria (in striatal cells) Delayed weight gain Lower protection against ROS |
[113] | |
| B6.129S4-Prkntm1Shn/J | X | X | Prkn KO mice | Increased extracellular dopamine concentration in the striatum Deficits in behavioral tests |
[114] | |
| Double-mutant “TwinkPark” mice, resulting from crossing B6.129S4-Prkntm1Shn/J line with Twinkledup/+ line | X | X | Prkn KO (enhanced in the substantia nigra) mice | Decrease of mitochondrial DNA Low mitochondrial function and membrane potential Neurobehavioral deficits |
[115] | |
| Crossing B6.129S4-Prkntm1Shn/J line with a Mcl-1 +/− line (n.s.n.) | X | X | Prkn KO + Mcl-1 +/− mice | Dopaminergic neuron loss Motor impairments |
[107] | |
| B6.129S4-Prkntm1Shn/J B6.129S4-Pink1tm1Shn/J |
X | X |
-Prkn KO mice -Pink1 KO mice |
Inflammation rescued by STING-mediated action | [116] | |
| B6.129S4-Prkntm1Shn/J B6.129S4-Pink1tm1Shn/J |
X | X |
-Prkn KO mice -Pink1 KO mice |
No repression of mitochondrial antigen presentation | [117] | |
| B6.129S4-Prkntm1Shn/J LEH-Pink1tm1sage |
X |
-Prkn KO mice -Pink1 KO rats |
Mitophagy of damaged mitochondria in axons requires PINK1 and Parkin | [118] | ||
| Crossing B6.129S4-Prkntm1Shn/J line and B6.129S4-Pink1tm1Shn/J line |
X |
Prkn/Pink1 double KO mice |
Higher levels of ATP synthase Denervated neuromuscular junctions |
[119] | ||
| B6.129S4-Prkntm1Shn/J B6.129S4-Pink1tm1Shn/J B6.Cg-Park7tm1Shn/J |
X | X |
-Prkn KO mice -Pink1 KO mice -Dj-1 KO mice |
Aberrant striatal synaptic plasticity in rodent models of autosomal recessive PD | [120] | |
| Crossing DASYN53 double-transgenic (tetO-SNCA*A53T) E2Cai/J line + DAT-PF-tTA) mice with B6.129S4-Prkntm1Shn/J line or with Pink1tm1Zhzh mutation line (n.s.n.) | X | X | Overexpressing human A53T-SNCA in DA neurons and KO for either Prkn or Pink1 |
Pervasive mitochondrial macroautophagy defects Dopamine neuron degeneration |
[121] | |
| B6;129-Pink1tm1Aub/J | X | Pink1 KO mice | Altered spontaneous EPSCs | [122] | ||
| B6;129-Pink1tm1Aub/J | X | Pink1 KO mice | Mitochondrial recruitment of parkin not affected | [123] | ||
| B6;129-Pink1tm1Aub/J | X | X | Pink1 KO mice | Progressive mitochondrial dysfunction in absence of neurodegeneration | [124] | |
| LRRK2 | B6.129X1(FVB)-Lrrk2tm1.1Cai/J | X | Lrrk2 KO mice | Alterations in protein synthesis Alterations in degradation pathways |
[125] | |
| B6.129X1(FVB)-Lrrk2tm1.1Cai/J | X | X | Lrrk2 KO mice | LRRK2 regulates synaptogenesis and dopamine receptor activation | [126] | |
| B6.129X1(FVB)-Lrrk2tm1.1Cai/J | X | Lrrk2 KO mice | LRRK2 regulates ER-Golgi export | [127] | ||
| B6.129X1(FVB)-Lrrk2tm1.1Cai/J | X | Lrrk2 KO mice | Neurons have more motile axonal and dendritic growth | [128] | ||
| C57BL/6-Lrrk2tm1Mjfa/J | X | X | Lrrk2 KO mice | LRRK2 modulates microglial phenotype and dopaminergic neurodegeneration | [129] | |
| C57BL/6-Lrrk2tm1Mjfa/J | X | Lrrk2 KO mice | Stress-Related Gastrointestinal Dysmotility | [130] | ||
| C57BL/6-Lrrk2tm1Mjfa/J | X | Lrrk2 KO mice | LRRK2 is required for Rip2 localization to DCVs | [131] | ||
| C57BL/6-Lrrk2tm1Mjfa/J | X | Lrrk2 KO mice | Significant increase of ceramide levels Direct effects on GBA1 |
[132] | ||
| B6;129-Lrrk2tm2.1Shn/J | X | X | Lrrk2 KO mice | Impairment of Autophagy | [133] | |
| B6;129-Lrrk2tm2.1Shn/J B6;129-Lrrk2tm3.1Shn/J |
X | Lrrk2 KO mice | Impairment of protein degradation pathways Apoptotic cell death |
[134] | ||
| C57BL/6-Lrrk2tm1.1Mjff/J | X | X | Lrrk2 KO mice | No obvious bone alteration phenotypes | [135] | |
| B6.Cg-Tg(Lrrk2)6Yue/J | X | X | Lrrk2 overexpressing mice | Autophagy suppression | [136] | |
| B6.FVB-Tg (LRRK2) WT1Mjfa/J | X | X | LRRK2 overexpressing mice | Behavioral hypoactivity Altered dopamine-dependent short-term plasticity |
[137] | |
| STOCK Tg (tetO-LRRK2*G2019S) E3Cai/J | X | G2019S-LRRK2 overexpressing mice | Perturbed homeostasis Altered neuronal morphogenesis |
[138] | ||
| B6.Cg-Tg (Lrrk2*G2019S) 2Yue/J | X | G2019S-LRRK2 overexpressing mice | Reduction in lysosomal pH Increased expression of lysosomal ATPases |
[139] | ||
| B6.FVB-Tg (LRRK2*G2019S) 1Mjfa/J | X | X | G2019S-LRRK2 overexpressing mice | Synapsis gain-of-function effect of the G2019S mutation |
[140] | |
| NTac:SD-Tg (LRRK2*G2019S) 571CJLi | X | X | G2019S-Lrrk2 overexpressing rats | Altered bone marrow myelopoiesis Peripheral myeloid cell differentiation |
[141] | |
| NTac:SD-Tg (LRRK2*G2019S) 571CJLi | X | X | G2019S-Lrrk2 overexpressing rats | Enhanced α-syn gene-induced neurodegeneration | [142] | |
| K-14Cre-positive Gbalnl/lnl | X | Gba KO mice (except in skin) | Reduced cerebral vascularization | [143] | ||
| PINK1 | B6.129S4-Pink1tm1Shn/J | X | Pink1 KO mice | Pink1 is not required for ubiquitination of mitochondrial proteins | [144] | |
| B6.129S4-Pink1tm1Shn/J | X | X | Pink1 KO mice | Reduced motor activity Slower locomotor activity time Absence of nigrostriatal dopamine loss |
[145] | |
| B6.129S4-Pink1tm1Shn/J | X | Pink1 KO mice | Impaired mitochondrial trafficking Fragmented mitochondria |
[146] | ||
| B6.129S4-Pink1tm1Shn/J | X | X | Pink1 KO mice | Hypersensitivity to MPTP-induced dopaminergic neuronal loss | [147] | |
| B6.129S4-Pink1tm1Shn/J | X | Pink1 KO mice | No significant change in Ca2+ currents | [148] | ||
| B6.129S4-Pink1tm1Shn/J | X | X | Pink1 KO mice | Pathological cardiac hypertrophy Greater levels of oxidative stress Impaired mitochondrial function |
[149] | |
| B6.129S4-Pink1tm1Shn/J | X | X | Pink1 KO mice | Impairments of corticostriatal LTP and LTD Impaired dopamine release |
[150] | |
| n.s.n. | X | Pink1 KO mice | Intestinal infection triggers Parkinson’s disease-like symptoms | [151] | ||
| Crossing B6;129-Pink1tm1Aub/J line with dOTC line | X | X | Pink1 KO mice overexpressing OTC in DA neurons | Enhanced neurodegeneration in a model of mitochondrial stress | [152] | |
| B6.129S4-Pink1tm1Shn/J B6.129S4-Prkntm1Shn/J |
X |
-Pink1 KO mice -Prkn KO mice |
Enhanced sensitivity to group II mGlu receptor activation | [153] | ||
| B6.129S4-Pink1tm1Shn/J B6.129S4-Prkntm1Shn/J |
X |
-Pink1 KO mice -Prkn KO mice |
Reduced mitochondria functions Altered mitophagy in macrophages |
[154] | ||
| FVB;129-Pink1tm1Aub Tg(Prnp-SNCA*A53T)AAub/J | X | A53T-SNCA overexpressing Pink1 KO mice |
Altered mitochondrial biogenesis | [155] | ||
| FVB;129-Pink1tm1Aub Tg(Prnp-SNCA*A53T)AAub/J | X | X | A53T-SNCA overexpressing Pink1 KO mice |
Exacerbated synucleinopathy | [156] | |
| FVB;129-Pink1tm1Aub Tg(Prnp-SNCA*A53T)AAub/J | X | X | A53T-SNCA overexpressing Pink1 KO mice |
Potentiation of neurotoxicity | [157] | |
| Atad3afl/fl Mx1CrePink1 −/− mice, resulting from crossing B6.129S4-Pink1tm1Shn/J line with Atad3afl/fl Mx1Cre line | X | X | Pink1 KO + conditional Atad3a KO mice | Aberrant stem-cell and progenitor homeostasis Pink1-dependent mitophagy |
[158] | |
| B6N.129S6(Cg)-Atp13a2tm1Pjsch/J | X | X | Atp13a2 KO mice | Autophagy impairment Reduced HDAC6 activity |
[159] | |
| B6N.129S6(Cg)-Atp13a2tm1Pjsch/J | X | Atp13a2 KO mice | Harmful gliosis | [160] | ||
| B6N.129S6(Cg)-Atp13a2tm1Pjsch/J | X | X | Atp13a2 KO mice | Neuronal ceroid lipofuscinosis Limited α-syn accumulation Sensorimotor deficits |
[161] |
n.s.n.: Non standard nomenclature.
In accordance with the leading role of α-syn in synucleinopathies such as PD [48], there are several transgenic animals expressing different versions of the SNCA gene and several studies on them. The results of these studies are interesting despite their bias as the link between α-syn and synucleinopathies is obvious. On the one hand, the lack of a gene (mouse SNCA knockout) produced viable animals with some mild abnormalities. It is the overexpression of the human gene that led to neural alterations. On the other hand, the expression of a non-phosphorylatable (S129A) mutation on an SNCA knockout background did not lead to any significant alteration in neural function. Neural alterations including dopaminergic neuronal death appeared upon overexpression of some gene mutants: often the mutant version included a phosphorylatable amino acid or, the other way around, eliminates a phosphorylatable residue. Findings using such proteins that may be differentially phosphorylated could link α-syn to the PD-associated genes that code for a kinase or a phosphatase. Also of interest was fact that multimers of the protein in samples from a knockout model attenuate synaptic vesicle recycling and that the α-syn spread correlated with dopaminergic neurodegeneration. Apparently unrelated to PD-associated genes was an alteration in the effect of neurotrophic factors that were detected in some of those animals. Finally, we would like to highlight that some of the studies reported alterations in mitochondrial dynamics or function. If mutated α-syn has more or less phosphorylatable sites, this may be related to an energy imbalance; in fact, kinase action requires ATP.
Animals that are KO for the Lrrk2 gene or overexpress human versions (mutant versions included) of this gene, consistently showed synaptogenetic deficiencies in intracellular vesicle trafficking and function and in protein processing. Interestingly, the G2019S mutation of the LRRK2 kinase produced neurodegeneration in a rodent model of human α-syn overexpression. The line expressing the G2019S mutation also displayed alterations in both homeostasis and neuronal morphogenesis.
Recent results from genetic and pharmacological experiments in a cell model showed that knocking down LRRK2 using a shRNA-based approach reduces oxidative stress by means of mitophagy [94]. A recent review uncovers the role of mitophagy in PD and lists the genes that may be involved in such phenomenon; many of the above-described PD-related genes may participate in mitophagy regulation (see [95] and references therein).
The number of transgenic animals related to other PD-associated genes is lower. There are, however, a significant number of studies on PRKN, PINK1, and DJ-1-related transgenic lines. Remarkably, Prkn knockout animals are resistant to the toxics usually used to produce dopaminergic neurodegeneration in wild-type rats or mice. In contrast, the Pink1 knockout showed hypersensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neuronal loss and to activation of some of glutamate receptors (both ionotropic and metabotropic), thus affecting excitatory neurotransmission. Studies with transgenic lines also show that the PRKN gene product is involved in protein processing, particularly via ubiquitination. In addition, the double mutant line, so called “TwinkPark” mice showed decreased mitochondrial function and altered membrane potential.
A common factor in all cases is mitochondrial affectation. It should be noted that of all the genes devoted to results on transgenic animals described so far in this section, the most related to mitochondria biogenesis and function is Pink1.
Results related to DJ-1 (PARK7) were quite diverse perhaps due to the fact that some rodent models were available >15 years ago. There is also a Drosophila melanogaster model in which expression of a mutant gene led to motor dysfunction related to oxidative stress. DJ-1 knockout mice displayed oxidative stress and altered regulation of autophagy that may be the consequence of previous findings: mitochondrial dysfunction and increased reactive oxygen species (ROS) production and higher sensitivity to excitotoxicity. At the CNS level, the animals have less dendritic arborization and reduced number of dendritic spines in the medium spiny neurons of the striatum.
Although the GBA glucocerebrosidase gene is a pretty new player in PD, it has been extensively studied for its involvement in hereditary Gaucher’s disease, which is caused by glucocerebrosides in lysosomes. When assessing the neurological abnormalities of the GBA transgenic lines an acceleration of the onset of PD traits was observed (e.g., motor impairment, and a downregulation of some neurotrophic factors).
Results from transgenic lines affecting Uchl1, Atp13a2, Pla2g6, SYNJ1 and FBXO7 genes are limited although they are included in Supplementary Table S1. In all cases the disturbances observed in the transgenic lines overlapped with the traits described earlier in this section. For instance, F-box protein 7 participates with Parkin in mitophagy-associated processes [162] and VPS35 participates in the transport of vesicles from mitochondria to peroxisomes [163].
7. α-Synuclein: Main Character or Supporting Actor?
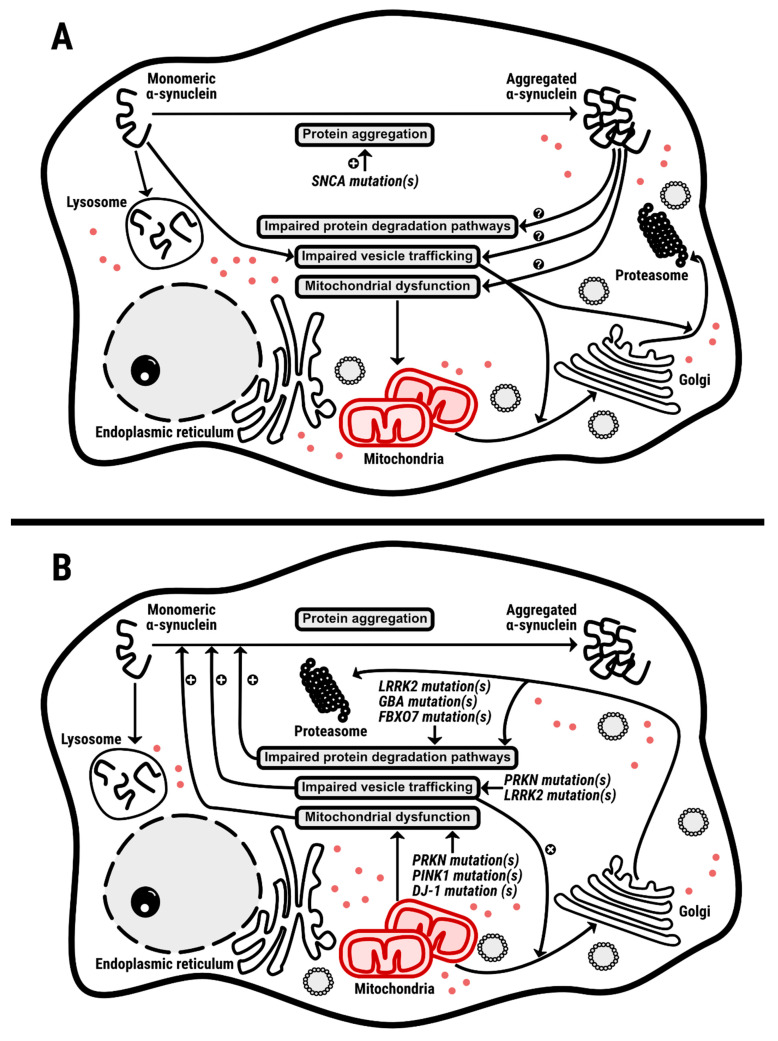
Of course, α-syn is an important factor in PD and in any other synucleinopathy. However, evidence from gene interaction studies indicates that there is no particular link with other genes related to familial PD. It should be noted the possibility that α-syn may be related to LRRK2. Although no direct evidence is yet available, it is suggested that that the kinase encoded by LRRK2, the physiological function of which is not known, could phosphorylate α-syn and such phosphorylation could have an impact in PD etiopathology (see [164,165]). If it were the cause of the disturbances leading, for instance, to dopaminergic denervation in PD, α-syn would be the main character. Many monogenic diseases appear early in childhood, but this is not the case in early onset PD caused by α-syn mutations. Therefore, it is likely that some α-syn mutants are aggravating the deterioration of mechanisms of homeostasis maintenance that occur with aging. These mechanisms would affect vesicular traffic, protein ubiquitination and protein processing/degradation, eventually leading to production of α-syn aggregates. It is necessary to point out that in familial forms of PD, aggregates occur for both mutated forms of α-syn, when the SNCA gene is affected and non-mutated α-syn, when the gene affected is another one of those in Table 1. One conclusion could be that α-syn is an important actor but not the main one. At the level of PD-related genes, there does not seem to be a main character because every gene product is necessary for the essential functions occurring in the dopaminergic neurons of the substantia nigra. Any mutation of those genes would lead to a gene product that would negatively affect cell function. Such an alteration never causes the disease to appear in the first years of life, thus suggesting that there is a progressive loss of function (failure to appropriately maintain homeostasis) consistent with the progressive character of the disease. Figure 3 displays two sides of an equation in which a mutant α-syn would affect mechanisms that, in turn, would lead to α-syn aggregation, and in which a mutant LRRK2, GBA, UCHL1, VPS35, PRKN, PINK1, ATP13A2, PLA2G6, DNAJC6, SYNJ1, DJ-1/PARK7 or FBXO7 genes would affect mechanisms that in time would affect the processing of nonmutated α-syn, thus leading to α-syn aggregation.
Figure 3.
Mechanisms of dyshomeostasis of dopaminergic neurons. Panel (A). Mutations in SNCA leads to aggregation of mutated α-synuclein that affects the mechanisms of protein handling and vesicle transport, thus dysbalancing mitochondrial dynamics and function. Panel (B). Mutations in genes that affect protein processing, vesicle transport and mitochondrial function leads to aggregation of non-mutated α-synuclein. Red dots represent ATP molecules, mainly synthesized in the mitochondria of neurons, that are needed for all the processes depicted in the schemes.
In agreement with the aberrant formation of α-syn aggregates, the vesicular transport or the proteasomal degradation mechanism is defective or overexposed. Although the neurotransmitters(s) involved are not known, the association of some PD-related genes to endocytosis indicates that signal transduction may regulate the processing of α-syn. Could dopaminergic signaling be involved? This is a possibility because of the unique vulnerability of the nigral neurons in PD.
Mitochondria are linked to all of the events mentioned above, either through mitogenesis and mitochondria-derived vesicles or through the provision of energy. In fact, a substantial amount of protein processing, from synthesis to ubiquitination-directed degradation, requires energy, primarily in the form of ATP. Energy is also required for vesicle/endosome sorting and trafficking. Neurons rely primarily on the breakdown of glucose to meet energy requirements, and anaerobic glycolysis would not be sufficient to support all neuronal processes. Therefore, complete oxidation of glucose is required since ATP is obtained mainly through the Krebs’ cycle and the electron-transport chain. As shown in Figure 3, mitochondria are center stage in all events involving the PD-related gene products. In fact, there are several studies linking proteins of Table 2 to mitochondrial action. One example from data obtained in Drosophila melanogaster demonstrated that loss-of-function PINK1 results in motor deficits due to neurodegeneration mediated by mitochondrial dysfunction. The authors concluded: “our genetic evidence clearly establishes that Parkin and PINK1 act in a common pathway in maintaining mitochondrial integrity and function in both muscles and dopaminergic neurons” [41]. Similar results were more recently obtained in a cell model and in primary cells obtained from patients. Loss-of-function of PINK1 in HeLa cells leads to altered mitochondrion function and morphology. Cells from patients with mutated PINK1 show altered mitochondrial morphology [42]. Another example is provided by [166], who showed that endoplasmic reticulum-mitochondrion interactions are regulated by LRRK2 and by PERK products via protein ubiquitination pathways. It is worth mentioning the MitoPark mice, in which a mutated gene for a mitochondrial transcription factor in dopaminergic neurons led to the defective expression of proteins encoded by mitochondrial DNA and a phenotype consistent with parkinsonism, including motor deficits and progressive neurodegeneration [167]. Because the MitoPark mice exhibited deficits in the electron transport chain, it raised the question of why nigral dopaminergic neurons or their mitochondria were more vulnerable than other neurons. A differential expression of α-syn in different neuronal populations may be a reasonable hypothesis. However, in our opinion, the dopamine metabolism should be taken into account because it adds extra oxidative stress to dopaminergic neurons. Indeed, dopamine leads to the production of a quinone, aminochrome (the precursor of neuromelanin), which significantly contributes to the dark color of the substantia nigra in humans. The reaction of dopamine with molecular oxygen can lead to other quinones and free radicals, an oxidative load that alters mitochondrial function and can produce autophagy in dopaminergic neurons [168,169].
Table 2.
Description of the products of the genes in Table 1. Function retrieved from https://string-db.org/ (accessed on 10 April 2021).
| Gene | Ensembl Identifier | Protein | Function (Information Obtained from STRING Data Base) |
|---|---|---|---|
| SNCA | ENSP00000338345 | α-synuclein | Involved in regulating dopamine release and transport. Induces the fibrillization of tau protein. Reduces neuronal responsiveness to different apoptotic stimuli, thus promoting a decreased caspase-3 activation. |
| LRRK2 | ENSP00000298910 | Leucine-rich repeat serine/threonine-protein kinase 2 | Regulates autophagy in a positive way by means of the calcium- dependent activation of the CaMKK/AMPK pathway, also involving the activation of nicotinic acid adenine dinucleotide phosphate (NAADP) receptors, increases in lysosomal pH, and release of Ca++ from lysosomes. |
| GBA | ENSP00000314508 | Glucosylceramidase beta | Involved in the hydrolization of glucocerebroside. Localized in lysosomes. |
| UCHL1 | ENSP00000284440 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | Deubiquitinating enzyme, generates ubiquitin monomers. Might prevent the degradation of monoubiquitin in lysosomes. Its expression is highly specific to neurons and to cells of the diffuse neuroendocrine system and their tumors. |
| VPS35 | ENSP00000299138 | Vacuolar protein sorting-associated protein 35 | Involved in autophagy. Is part of the retromer cargo-selective complex (CSC), which is responsible for transporting select cargo proteins between vesicular structures (e.g., endosomes, lysosomes, vacuoles) and the Golgi apparatus. |
| PRKN | ENSP00000355865 | E3 ubiquitin-protein ligase parkin | Ubiquitin ligase; covalently binds ubiquitin residues onto proteins. Involved in the removal of abnormally folded or damaged proteins thanks to ‘Lys-63’-linked polyubiquitination of misfolded proteins. |
| PINK1 | ENSP00000364204 | Serine/threonine-protein kinase PINK1 | Localized in mitochondria. Protects cells against stress-induced mitochondrial dysfunction by phosphorylating mitochondrial proteins. By means of activation and translocation of PRKN participates in the clearance of damaged mitochondria via selective autophagy (mitophagy). |
| ATP13A2 | ENSP00000327214 | Cation-transporting ATPase 13A2 | ATPase involved in the transport of divalent transition metal cations and the maintenance of neuronal integrity. It is necessary for a correct lysosomal and mitochondrial maintenance. |
| PLA2G6 | ENSP00000333142 | 85/88 kDa calcium-independent phospholipase A2 | Involved in the release of fatty acids from phospholipids. Implicated in normal phospholipid remodeling. It has also been involved in NO- or vasopressin-induced arachidonic acid release and in leukotriene and prostaglandin production. |
| DNAJC6 | ENSP00000360108 | Putative tyrosine-protein phosphatase auxilin | Promotes uncoating of clathrin-coated vesicles by recruiting HSPA8/HSC70 to clathrin-coated vesicles. Involved in clathrin-mediated endocytosis in neurons. |
| SYNJ1 | ENSP00000409667 | Synaptojanin-1 | Phosphoinositide phosphatase, regulates levels of membrane phosphatidylinositol-4,5-bisphosphate (PIP2). Involved in the rearrangement of actin filaments downstream of tyrosine kinase and ASH/GRB2 by means of hydrolyzing PIP2 bound to actin regulatory proteins. |
| DJ-1/ PARK7 | ENSP00000418770 | Protein/nucleic acid deglycase DJ-1 | Under an oxidative condition, via its chaperone activity, inhibits the aggregation of α-synuclein, thus functioning as a redox-sensitive chaperone and as a sensor for oxidative stress. Deglycates proteins and nucleotides, and the Maillard adducts formed between amino groups of proteins or nucleotides and reactive carbonyl groups of glyoxals. |
| FBXO7 | ENSP00000266087 | F-box only protein 7 | Part of a SCF (SKP1-CUL1-F- box protein) E3 ubiquitin-protein ligase complex involved in protein ubiquitination. Role in the clearance of damaged mitochondria (mitophagy). |
8. Concluding Remarks
Information available from genes related to familial PD led to a scenario that may be useful for explaining neurodegeneration in sporadic cases. Apart from a potential link between the kinase coded by LRRK2 and the phosphorylation of α-Syn, which is not yet substantiated, there is no direct relationship between the gene for this protein (α-Syn) and all the other genes displayed in Table 1. Hence, a common factor in familial and sporadic cases may be a progressive loss of efficacy in the mechanisms of vesicle traffic and protein handling, especially in degradation by the proteasome and the lysosome. The loss is quicker in familial cases as there is an excess burden due to difficulties in processing mutant forms of α-Syn or to difficulties in processing α-Syn by mutant components of the protein-processing machinery. For those processes, neuronal energy in the form of ATP is dependent of the full oxidation of glucose (i.e., involvement of mitochondrial Krebs’s cycle and electron chain transport). The higher vulnerability of dopaminergic neurons may come from the need of appropriate α-Syn processing and also from the oxidative stress produced by dopamine metabolism. It is reasonable to speculate that mitochondria copes with the supply of additional energy and with oxidative stress to the point where the demands for energy and antioxidant actions can no longer be satisfied, and cell death occurs: earlier in familial cases but later in sporadic cases. Hence, it can be speculated that any action to help mitochondria would be useful for preventing neurodegeneration. In general, to improve mitochondrial performance, coenzyme Q is useful; in fact, there is a correlation between oxidative stress and the deficit of this molecule in centenarians [170,171,172]. However, better options for improving the antioxidant machinery in the central nervous system are needed. The discovery of G protein-coupled receptors (GPCRs) in the mitochondria of neurons [173,174,175] may end up helping target those receptors to reduce oxidative burden or increase mitochondrial performance. Drugs acting on GPCRs constitute 35–45% of all approved therapeutic drugs and antagonists of receptors that are targets of neuroprotection (e.g., the adenosine A2A receptor) may help. Could it be that the decreased risk of suffering from neurodegenerative diseases after consuming the adenosine-receptor antagonists caffeine (coffee and cola drinks) and theophylline (tea) [8,176,177,178,179,180,181,182,183,184,185,186,187,188,189] is due to an effect on neuronal mitochondria?
Acknowledgments
Work supported by a grant (#RTI2018-09204-B-I00) from the Spanish Ministerio de Ciencia, Innovación y Universidades (MCIU) and Spanish Agencia Estatal de Investigación (AEI); it includes UE FEDER funds. The research group of the University of Barcelona is considered of excellence (grup consolidat #2017 SGR 1497) by the Regional Catalonian Government, which does not provide any specific funding for reagents or for payment of services or Open Access fees).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22094643/s1, Figure S1: STRING analysis of connections of either SNCA (A), GBA (B), FBXO7 (C), ATP13A2 (D) and UCHL1 (E). Table S1: Findings in animal models related to Gba, Uchl1, Vps35, Atp13a2, Pla2g6, Dnajc6, Synj1, DJ-1/Park7 and Fbxo7.
Author Contributions
R.F. and A.P. designed the paper. R.F., R.R.-S., G.N., A.P. and I.R.-R. retrieved information for the literature, selected the papers and summarized their data. The tables were constructed by A.P., I.R.-R. and R.R.-S. The figures were constructed by R.F. and R.R.-S., R.F. and G.N. wrote the first draft. All co-authors contributed equally to the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant (#RTI2018-09204-B-I00) from the Spanish Ministerio de Ciencia, Innovación y Universidades (MCIU) and Spanish Agencia Estatal de Investigación (AEI); it includes UE FEDER funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lesage S., Lunati A., Houot M., Ben Romdhan S., Clot F., Tesson C., Mangone G., Toullec B.L., Courtin T., Larcher K., et al. Characterization of Recessive Parkinson Disease in a Large Multicenter Study. Ann. Neurol. 2020;88:843–850. doi: 10.1002/ana.25787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng H., Wang P., Jankovic J. The genetics of Parkinson disease. Ageing Res. Rev. 2018;42:72–85. doi: 10.1016/j.arr.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Emamzadeh F.N., Surguchov A. Parkinson’s disease: Biomarkers, treatment, and risk factors. Front. Neurosci. 2018;12:612. doi: 10.3389/fnins.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oñatibia-Astibia A., Franco R., Martínez-Pinilla E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017;61:1600670. doi: 10.1002/mnfr.201600670. [DOI] [PubMed] [Google Scholar]
- 5.Simon D.K., Wu C., Tilley B.C., Lohmann K., Klein C., Payami H., Wills A.M., Aminoff M.J., Bainbridge J., Dewey R., et al. Caffeine, creatine, GRIN2A and Parkinson’s disease progression. J. Neurol. Sci. 2017;375:355–359. doi: 10.1016/j.jns.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sipetic S.B., Vlajinac H.D., Maksimovic J.M., Marinkovic J.M., Dzoljic E.D., Ratkov I.S., Kostic V.S. Cigarette smoking, coffee intake and alcohol consumption preceding Parkinson’s disease: A case-control study. Acta Neuropsychiatr. 2012;24:109–114. doi: 10.1111/j.1601-5215.2011.00593.x. [DOI] [PubMed] [Google Scholar]
- 7.Ross G.W., Petrovitch H. Current Evidence for Neuroprotective Effects of Nicotine and Caffeine Against Parkinsons Disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ross G.W., RD A., Petrovitch H., Al E., Abbott R.D., Petrovitch H., Morens D.M., Grandinetti A., Tung K.H., Tanner C.M., et al. Association of coffee and caffeine intake with the risk of parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez-Jiménez F.J., Mateo D., Giméanez-Roldan S. Premorbid smoking, alcohol consumption, and coffee drinking habits in Parkinson’s disease: A case-control study. Mov. Disord. 1992;7:339–344. doi: 10.1002/mds.870070407. [DOI] [PubMed] [Google Scholar]
- 10.Bronstein J., Carvey P., Chen H., Cory-Slechta D., DiMonte D., Duda J., English P., Goldman S., Grate S., Hanssen J., et al. Meeting report: Consensus Statement—Parkinson’s disease and the environment: Collaborative on health and the environment and Parkinson’s action network (CHE PAN) conference 26–28 June 2007. Environ. Health Perspect. 2009;117:117–121. doi: 10.1289/ehp.11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J.-F. Adenosine receptor control of cognition in normal and disease. Int. Rev. Neurobiol. 2014;119:257–307. doi: 10.1016/B978-0-12-801022-8.00012-X. [DOI] [PubMed] [Google Scholar]
- 12.Elbaz A., Carcaillon L., Kab S., Moisan F. Epidemiology of Parkinson’s disease. Rev. Neurol. 2016;172:14–26. doi: 10.1016/j.neurol.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Blauwendraat C., Nalls M.A., Singleton A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19:170–178. doi: 10.1016/S1474-4422(19)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birkmayer W., Hornykiewicz O. The L-dihydroxyphenylalanine (L-DOPA) effect in Parkinson’s syndrome in man: On the pathogenesis and treatment of Parkinson akinesis. Arch. Psychiatr. Nervenkr. Z. Gesamte Neurol. Psychiatr. 1962;203:560–574. doi: 10.1007/BF00343235. [DOI] [PubMed] [Google Scholar]
- 15.Hornykiewicz O. Die topische Lokalization und des Verhalten von Noradrenalin und Dopamin (3-Hydroxytyramin) in der Substantia nigra der normalen und Parkinson Kranken Menschen. Wien. Klin. Wochschr. 1963;75:309–312. [PubMed] [Google Scholar]
- 16.Birkmayer W., Hornykiewicz O. Additional experimental studies on L-DOPA in Parkinson’s syndrome and reserpine parkinsonism. Arch. Psychiatr. Nervenkr. 1964;206:367–381. doi: 10.1007/BF00341704. [DOI] [PubMed] [Google Scholar]
- 17.Holzer G., Hornykiewicz O. Über den Dopamin-(Hydroxytyramin-)Stoffwechsel im Gehirn der Ratte. Naunyn-Schmiedeberg’s Arch. Exp. Pathol. Pharmakol. 1959;237:27–33. [PubMed] [Google Scholar]
- 18.Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J. Neural Transm. Suppl. 2006;70:9–15. doi: 10.1007/978-3-211-45295-0_3. [DOI] [PubMed] [Google Scholar]
- 19.Olanow C.W., Agid Y., Mizuno Y., Albanese A., Bonuccelli U., Bonucelli U., Damier P., De Yebenes J., Gershanik O., Guttman M., et al. Levodopa in the treatment of Parkinson’s disease: Current controversies. Mov. Disord. 2004;19:997–1005. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- 20.Walter B.L., Vitek J.L. Surgical treatment for Parkinson’s disease. Lancet Neurol. 2004;3:719–728. doi: 10.1016/S1474-4422(04)00934-2. [DOI] [PubMed] [Google Scholar]
- 21.Conley S.C., Kirchner J.T. Medical and surgical treatment of Parkinson’s disease: Strategies to slow symptom progression and improve quality of life. Postgrad. Med. 1999;106:41–52. doi: 10.3810/pgm.1999.08.648. [DOI] [PubMed] [Google Scholar]
- 22.Durif F. Treating and preventing levodopa-induced dyskinesias: Current and future strategies. Drugs Aging. 1999;14:337–345. doi: 10.2165/00002512-199914050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R., Lozano A.M., Montgomery E., Lang A.E. Pallidotomy and deep brain stimulation of the pallidum and subthalamic nucleus in advanced Parkinson’s disease. Mov. Disord. 1998;13(Suppl. 1):73–82. [PubMed] [Google Scholar]
- 24.Arle J.E., Alterman R.L. Surgical options in Parkinson’s disease. Med. Clin. N. Am. 1999;83:483–498. doi: 10.1016/S0025-7125(05)70115-2. [DOI] [PubMed] [Google Scholar]
- 25.Limousin-Dowsey P., Pollak P., Blercom N., Krack P., Benazzouz A., Benabid A.-L. Thalamic, subthalamic nucleus and internal pallidum stimulation in Parkinson’s disease. J. Neurol. 1999;246:II42–II45. doi: 10.1007/BF03161080. [DOI] [PubMed] [Google Scholar]
- 26.Gross C.E., Boraud T., Guehl D., Bioulac B., Bezard E. From experimentation to the surgical treatment of Parkinson’s disease: Prelude or suite in basal ganglia research? Prog. Neurobiol. 1999;59:509–532. doi: 10.1016/S0301-0082(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 27.Gross R.E., Lozano A.M. Advances in neurostimulation for movement disorders. Neurol. Res. 2000;22:247–258. doi: 10.1080/01616412.2000.11740667. [DOI] [PubMed] [Google Scholar]
- 28.Caparros-Lefebvre D., Blond S., Vermersch P., Pecheux N., Guieu J.D., Petit H. Chronic thalamic stimulation improves tremor and levodopa induced dyskinesias in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1993;56:268–273. doi: 10.1136/jnnp.56.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2012;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 30.McKeith I.G., Burn D. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol. Clin. 2000;18:865–883. doi: 10.1016/S0733-8619(05)70230-9. [DOI] [PubMed] [Google Scholar]
- 31.McCann H., Stevens C.H., Cartwright H., Halliday G.M. α-Synucleinopathy phenotypes. Park. Relat. Disord. 2014;20:S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 32.Smeyne R.J., Noyce A.J., Byrne M., Savica R., Marras C. Infection and risk of Parkinson’s disease. J. Parkinsons. Dis. 2021;11:31–43. doi: 10.3233/JPD-202279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunati A., Lesage S., Brice A. The genetic landscape of Parkinson’s disease. Rev. Neurol. 2018;174:628–643. doi: 10.1016/j.neurol.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Puschmann A. New Genes Causing Hereditary Parkinson’s Disease or Parkinsonism. Curr. Neurol. Neurosci. Rep. 2017;17:1–11. doi: 10.1007/s11910-017-0780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas A.L., Warms J.V., Hershko A., Rose I.A. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J. Biol. Chem. 1982;257:2543–2548. doi: 10.1016/S0021-9258(18)34958-5. [DOI] [PubMed] [Google Scholar]
- 36.Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J. Cell. Biochem. 1984;24:27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- 37.Hershko A., Leshinsky E., Ganoth D., Heller H. ATP-dependent degradation of ubiquitin-protein conjugates. Proc. Natl. Acad. Sci. USA. 1984;81:1619–1623. doi: 10.1073/pnas.81.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonifati V., Dekker M.C.J., Vanacore N., Fabbrini G., Squitieri F., Marconi R., Antonini A., Brustenghi P., Dalla Libera A., De Mari M., et al. Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK. Neurol. Sci. 2002;23:59–60. doi: 10.1007/s100720200069. [DOI] [PubMed] [Google Scholar]
- 39.Moore D.J., Zhang L., Troncoso J., Lee M.K., Hattori N., Mizuno Y., Dawson T.M., Dawson V.L. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum. Mol. Genet. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- 40.Clark I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 41.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.M., et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 42.Exner N., Treske B., Paquet D., Holmström K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H.H., Gasser T., et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith W.W., Pei Z., Jiang H., Moore D.J., Liang Y., West A.B., Dawson V.L., Dawson T.M., Ross C.A. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuspiel M., Schauss A.C., Braschi E., Zunino R., Rippstein P., Rachubinski R.A., Andrade-Navarro M.A., McBride H.M. Cargo-Selected Transport from the Mitochondria to Peroxisomes Is Mediated by Vesicular Carriers. Curr. Biol. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 45.Lee D.W., Zhao X., Yim Y.I., Eisenberg E., Greene L.E. Essential role of cyclin-G-associated kinase (auxilin-2) in developing and mature mice. Mol. Biol. Cell. 2008;19:2766–2776. doi: 10.1091/mbc.e07-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pishvaee B., Costaguta G., Yeung B.G., Ryazantsev S., Greener T., Greene L.E., Eisenberg E., McCaffery J.M., Payne G.S. A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat. Cell Biol. 2000;2:958–963. doi: 10.1038/35046619. [DOI] [PubMed] [Google Scholar]
- 47.Toret C.P., Lee L., Sekiya-Kawasaki M., Drubin D.G. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic. 2008;9:848–859. doi: 10.1111/j.1600-0854.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 48.Surguchev A.A., Surguchov A. Synucleins and gene expression: Ramblers in a crowd or cops regulating traffic? Front. Mol. Neurosci. 2017;10:224. doi: 10.3389/fnmol.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Austin S.A., Floden A.M., Murphy E.J., Combs C.K. α-Synuclein Expression Modulates Microglial Activation Phenotype. J. Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golovko M.Y., Faergeman N.J., Cole N.B., Castagnet P.I., Nussbaum R.L., Murphy E.J. α-Synuclein Gene Deletion Decreases Brain Palmitate Uptake and Alters the Palmitate Metabolism in the Absence of R -Synuclein Palmitate Binding. Biochemistry. 2005;44:8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- 51.Ellis C.E., Murphy E.J., Mitchell D.C., Golovko M.Y., Scaglia F., Barcelo G.C., Nussbaum R.L. Mitochondrial Lipid Abnormality and Electron Transport Chain Impairment in Mice Lacking α-Synuclein. Mol. Cell. Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabin D.E., Shimazu K., Murphy D., Cole N.B., Gottschalk W., Mcilwain K.L., Orrison B., Chen A., Ellis C.E., Paylor R., et al. Synaptic Vesicle Depletion Correlates with Attenuated Synaptic α-Synuclein. J. Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pathak D., Berthet A., Bendor J.T., Yu K., Sellnow R.C., Orr L., Nguyen M.K., Edwards R.H., Manfredsson F.P., Nakamura K. Loss of α-Synuclein Does Not Affect Mitochondrial Bioenergetics in Rodent Neurons. Eneuro. 2017;4 doi: 10.1523/ENEURO.0216-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beatman E.L., Massey A., Shives K.D., Burrack K.S., Chamanian M., Morrison T.E. α-Synuclein Expression Restricts RNA Viral Infections in the Brain. J. Virol. 2016;90:2767–2782. doi: 10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renella R., Schlehe J.S., Selkoe D.J., Williams D.A., LaVoie M.J. Genetic deletion of the GATA1-regulated protein α-synuclein reduces oxidative stress and nitric oxide synthase levels in mature erythrocytes. Am. J. Hematol. 2014;89:974–977. doi: 10.1002/ajh.23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paine S.M.L., Anderson G., Bedford K., Lawler K., Mayer R.J., Lowe J., Bedford L. Pale Body-Like Inclusion Formation and Neurodegeneration following Depletion of 26S Proteasomes in Mouse Brain Neurones are Independent of α-Synuclein. PLoS ONE. 2013;8:e54711. doi: 10.1371/journal.pone.0054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scott D., Roy S. α-Synuclein Inhibits Intersynaptic Vesicle Mobility and Maintains Recycling-Pool Homeostasis. J. Neurosci. 2012;32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kokhan V.S., Afanasyeva M.A., Van G.I. α-Synuclein knockout mice have cognitive impairments. Behav. Brain Res. 2012;231:226–230. doi: 10.1016/j.bbr.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Cano-jaimez M., Pérez-sánchez F., Milán M., Buendía P., Ambrosio S., Fariñas I. Neurobiology of Disease Vulnerability of peripheral catecholaminergic neurons to MPTP is not regulated by α-synuclein. Neurobiol. Dis. 2010;38:92–103. doi: 10.1016/j.nbd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Klivenyi P., Siwek D., Gardian G., Yang L., Starkov A., Cleren C., Ferrante R.J., Kowall N.W., Abeliovich A., Beal M.F. Mice lacking α-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Abeliovich A., Schmitz Y., Farin I., Choi-lundberg D., Ho W., Castillo P.E., Shinsky N., Manuel J., Verdugo G., Armanini M., et al. Mice Lacking α-Synuclein Display Functional Deficits in the Nigrostriatal Dopamine System. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 62.Peña-Oliver Y., Buchman V.L., Dalley J.W., Robbins T.W., Schumann G., Ripley T.L., King S.L., Stephens D.N. Deletion of α-synuclein decreases impulsivity in mice. Genes Brain Behav. 2012;11:137–146. doi: 10.1111/j.1601-183X.2011.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelkonen A., Yavich L. Neuroscience Letters Neuromuscular pathology in mice lacking α-synuclein. Neurosci. Lett. 2011;487:350–353. doi: 10.1016/j.neulet.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 64.Chadchankar H., Ihalainen J., Tanila H., Yavich L. Decreased reuptake of dopamine in the dorsal striatum in the absence of α-synuclein. Brain Res. 2011;1382:37–44. doi: 10.1016/j.brainres.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 65.Al-Wandi A., Ninkina N., Millership S., Williamson S.J.M., Jones P.A., Buchman V.L. Absence of α-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol. Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senior S.L., Ninkina N., Deacon R., Bannerman D., Vladimir L., Cragg S.J., Wade-martins R. Europe PMC Funders Group Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both α-synuclein and gamma- synuclein. J. Neurosci. 2011;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anwar S., Peters O., Millership S., Ninkina N., Doig N., Connor-robson N., Threlfell S., Kooner G., Deacon R.M., Bannerman D.M., et al. Functional Alterations to the Nigrostriatal System in Mice Lacking All Three Members of the Synuclein Family. J. Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vargas K.J., Makani S., Davis T., Westphal C.H., Castillo P.E., Chandra S.S. Synucleins Regulate the Kinetics of Synaptic Vesicle Endocytosis. J. Neurosci. 2014;34:9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greten-Harrison B., Polydoro M., Morimoto-Tomita M., Diao L., Williams A.M. αβγ -Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. USA. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vargas K.J., Schrod N., Davis T., Fernandez-Busnadiego R., Taguchi Y.V., Laugks U., Lucic V., Chandra S.S. Synucleins Have Multiple Effects on Presynaptic Architecture. Cell Rep. 2017;18:161–173. doi: 10.1016/j.celrep.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su S., Zhang Z., Liu X., Manfredsson F.P., Benskey M.J., Cao X., Xu J. TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2017;114:10773–10778. doi: 10.1073/pnas.1713969114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarafian T.A., Ryan C.M., Souda P., Masliah E., Kar U.K., Vinters H.V., Mathern G.W., Faull K.F., Whitelegge J.P., Watson J.B. Impairment of Mitochondria in Adult Mouse Brain Overexpressing Predominantly Full-Length, N-Terminally Acetylated Human α-Synuclein. PLoS ONE. 2013;8:e63557. doi: 10.1371/journal.pone.0063557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watson J.B., Hatami A., David H., Masliah E., Roberts K., Evans C.E., Levine M.S. Aleterations in corticostriatal synaptic plasticity in mice overexpressing human α-synuclein. NSC. 2009;159:501–513. doi: 10.1016/j.neuroscience.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reznichenko L., Cheng Q., Nizar K., Gratiy S.L., Saisan P.A., Rockenstein E.M., Patrick C., Spencer B., Desplats P., Dale A.M., et al. In Vivo Alterations in Calcium Buffering Capacity in Transgenic Mouse Model of Synucleinopathy. J. Neurosci. 2012;32:9992–9998. doi: 10.1523/JNEUROSCI.1270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siebert H., Kahle P.J., Kramer M.L., Isik T., Schlüter O.M., Schulz-Schaeffer W.J., Brück W. Over-expression of α-synuclein in the nervous system enhances axonal degeneration after peripheral nerve lesion in a transgenic mouse strain. J. Neurochem. 2010;114:1007–1018. doi: 10.1111/j.1471-4159.2010.06832.x. [DOI] [PubMed] [Google Scholar]
- 76.Janezic S., Threlfell S., Dodson P.D., Dowie M.J., Taylor T.N., Potgieter D., Parkkinen L., Senior S.L., Anwar S., Ryan B., et al. Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc. Natl. Acad. Sci. USA. 2013;110:E4016–E4025. doi: 10.1073/pnas.1309143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunn B.H.M., Vingill S., Threlfell S., Bannerman D.M., Cragg S.J., Wade-martins R., Hunn B.H.M., Vingill S., Threlfell S., Alegre-abarrategui J., et al. Impairment of Macroautophagy in Dopamine Neurons Has Opposing Effects on Parkinsonian Pathology and Behavior Article Impairment of Macroautophagy in Dopamine Neurons Has Opposing Effects on Parkinsonian Pathology and Behavior. Cell Rep. 2019;29:920–931.e7. doi: 10.1016/j.celrep.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L., Das U., Scott D.A., Tang Y., McLean P.J., Roy S. α-Synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 2014;24:2319–2326. doi: 10.1016/j.cub.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Escobar V.D., Kuo Y.-M., Orrison B.M., Giasson B.I., Nussbaum R.L. Transgenic mice expressing S129 phosphorylation mutations in α-synuclein. Neurosci. Lett. 2014;563:96–100. doi: 10.1016/j.neulet.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuo Y., Li Z., Jiao Y., Gaborit N., Pani A.K., Orrison B.M., Bruneau B.G., Giasson B.I., Smeyne R.J., Gershon M.D., et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated α-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 2010;19:1633–1650. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao H., Kotzbauer P.T., Uryu K., Leight S., Trojanowski J.Q., Lee V.M. Neuroinflammation and Oxidation / Nitration of α-Synuclein Linked to Dopaminergic Neurodegeneration. J. Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor T.N., Potgieter D., Anwar S., Senior S.L., Janezic S., Threlfell S., Ryan B., Parkkinen L., Deltheil T., Cioroch M., et al. Region-specific deficits in dopamine, but not norepinephrine, signaling in a novel A30P α-synuclein BAC transgenic mouse. Neurobiol. Dis. 2014;62:193–207. doi: 10.1016/j.nbd.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurz A., Double K.L., Lastres-becker I., Tozzi A., Tantucci M., Nuber S., Schlaudraff F., Bockhart V., Bonin M., Ferna J. A53T-α-Synuclein Overexpression Impairs Dopamine Signaling and Striatal Synaptic Plasticity in Old Mice. PLoS ONE. 2010;5:e11464. doi: 10.1371/journal.pone.0011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gispert S., Turco D., Garrett L., Chen A., Bernard D.J., Hamm-clement J., Korf H., Deller T., Braak H., Auburger G., et al. Transgenic mice expressing mutant A53T human α-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol. Cell. Neurosci. 2003;24:419–429. doi: 10.1016/S1044-7431(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 85.Yamasaki T., Fujinaga M., Kawamura K., Furutsuka K., Nengaki N., Shimoda Y., Shiomi S., Takei M., Hashimoto H., Yui J., et al. Dynamic Changes in Striatal mGluR1 But Not mGluR5 during Pathological Progression of Parkinson’s Disease in Human Alpha-Synuclein A53T Transgenic Rats: A Multi-PET Imaging Study. J. Neurosci. 2016;36:375–384. doi: 10.1523/JNEUROSCI.2289-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luk K.C., Kehm V.M., Zhang B., Brien P.O., Trojanowski J.Q., Lee V.M.Y. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cannon J.R., Geghman K.D., Tapias V., Sew T., Dail M.K., Li C., Greenamyre J.T. Expression of human E46K-mutated α-synuclein in BAC-transgenic rats replicates early-stage Parkinson’s disease features and enhances vulnerability to mitochondrial impairment. Exp. Neurol. 2013;240:44–56. doi: 10.1016/j.expneurol.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimshek D.R., Schweizer T., Schmid P., Van Der Putten P.H. Excess α-synuclein worsens disease in mice lacking ubiquitin carboxy-terminal hydrolase L1. Sci. Rep. 2012;2:1–8. doi: 10.1038/srep00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martín-Clemente B., Alvarez-Castelao B., Mayo I., Sierra A.B., Díaz V., Milán M., Fariñas I., Gómez-Isla T., Ferrer I., Castaño J.G. α-Synuclein expression levels do not significantly affect proteasome function and expression in mice and stably transfected PC12 cell lines. J. Biol. Chem. 2004;279:52984–52990. doi: 10.1074/jbc.M409028200. [DOI] [PubMed] [Google Scholar]
- 90.Sharon R., Bar-joseph I., Mirick G.E., Serhan C.N., Selkoe D.J. Altered Fatty Acid Composition of Dopaminergic Neurons Expressing α-Synuclein and Human Brains with α-Synucleinopathies. J. Biol. Chem. 2003;278:49874–49881. doi: 10.1074/jbc.M309127200. [DOI] [PubMed] [Google Scholar]
- 91.Nemani V.M., Lu W., Berge V., Nakamura K., Onoa B., Lee M.K., Chaudhry F.A., Nicoll R.A., Edwards R.H. Increased Expression of α-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim S., Kwon S., Kam T., Dawson V.L., Dawson T.M., Ko H.S., Kim S., Kwon S., Kam T., Panicker N., et al. Transneuronal Propagation of Pathologic a -Synuclein from the Gut to the Brain Models Parkinson’ s Disease Article Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’ s Disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng C., Gathagan R.J., Covell D.J., Medellin C., Stieber A., Robinson J.L., Zhang B., Pitkin R.M., Olufemi M.F., Luk K.C., et al. α -synuclein strains in α -synucleinopathies. Nature. 2018;557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin J., Zheng X., Zhang Z., Zhuge J., Shao Z., Huang C., Jin J., Chen X., Chen Y., Wu Y., et al. Inhibition of LRRK2 restores parkin-mediated mitophagy and attenuates intervertebral disc degeneration. Osteoarthr. Cartil. 2021;29:579–591. doi: 10.1016/j.joca.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Wang X.L., Feng S.T., Wang Y.T., Yuan Y.H., Li Z.P., Chen N.H., Wang Z.Z., Zhang Y. Mitophagy, a Form of Selective Autophagy, Plays an Essential Role in Mitochondrial Dynamics of Parkinson’s Disease. Cell. Mol. Neurobiol. 2021 doi: 10.1007/s10571-021-01039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Hebron M., Shi W., Lonskaya I., Moussa C.E.H. Ubiquitin specific protease-13 independently regulates parkin ubiquitination and α-synuclein clearance in alpha-synucleinopathies. Hum. Mol. Genet. 2019;28:548–560. doi: 10.1093/hmg/ddy365. [DOI] [PubMed] [Google Scholar]
- 97.Cartelli D., Amadeo A., Calogero A.M., Casagrande F.V.M., De Gregorio C., Gioria M., Kuzumaki N., Costa I., Sassone J., Ciammola A., et al. Parkin absence accelerates microtubule aging in dopaminergic neurons. Neurobiol. Aging. 2018;61:66–74. doi: 10.1016/j.neurobiolaging.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 98.Peker N., Donipadi V., Sharma M., McFarlane C., Kambadur R. Loss of Parkin impairs mitochondrial function and leads to muscle atrophy. Am. J. Physiol. Cell Physiol. 2018;315:C164–C185. doi: 10.1152/ajpcell.00064.2017. [DOI] [PubMed] [Google Scholar]
- 99.Williams E.T., Glauser L., Tsika E., Jiang H., Islam S., Moore D.J. Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum. Mol. Genet. 2018;27:3189–3205. doi: 10.1093/hmg/ddy224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh K., Han K., Tilve S., Wu K., Geller H.M., Sack M.N. Parkin targets NOD2 to regulate astrocyte endoplasmic reticulum stress and inflammation. Glia. 2018;66:2427–2437. doi: 10.1002/glia.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hwang C.J., Kim Y.E., Son D.J., Park M.H., Choi D.Y., Park P.H., Hellström M., Han S.B., Oh K.W., Park E.K., et al. Parkin deficiency exacerbate ethanol-induced dopaminergic neurodegeneration by P38 pathway dependent inhibition of autophagy and mitochondrial function. Redox Biol. 2017;11:456–468. doi: 10.1016/j.redox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kabayama H., Tokushige N., Takeuchi M., Kabayama M., Fukuda M., Mikoshiba K. Parkin promotes proteasomal degradation of synaptotagmin IV by accelerating polyubiquitination. Mol. Cell. Neurosci. 2017;80:89–99. doi: 10.1016/j.mcn.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Lin M.Y., Cheng X.T., Tammineni P., Xie Y., Zhou B., Cai Q., Sheng Z.H. Releasing Syntaphilin Removes Stressed Mitochondria from Axons Independent of Mitophagy under Pathophysiological Conditions. Neuron. 2017;94:595–610.e6. doi: 10.1016/j.neuron.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pickrell A.M., Huang C.H., Kennedy S.R., Ordureau A., Sideris D.P., Hoekstra J.G., Harper J.W., Youle R.J. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron. 2015;87:371–381. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kubli D.A., Zhang X., Lee Y., Hanna R.A., Quinsay M.N., Nguyen C.K., Jimenez R., Petrosyans S., Murphy A.N., Gustafsson Å.B. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J. Biol. Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sul J.W., Park M.Y., Shin J., Kim Y.R., Yoo S.E., Kong Y.Y., Kwon K.S., Lee Y.H., Kim E. Accumulation of the parkin substrate, FAF1, plays a key role in the dopaminergic neurodegeneration. Hum. Mol. Genet. 2013;22:1558–1573. doi: 10.1093/hmg/ddt006. [DOI] [PubMed] [Google Scholar]
- 107.Ekholm-Reed S., Baker R., Campos A.R., Stouffer D., Henze M., Wolf D.A., Loring J.F., Thomas E.A., Reed S.I. Reducing Mcl-1 gene dosage induces dopaminergic neuronal loss and motor impairments in Park2 knockout mice. Commun. Biol. 2019;2:1–7. doi: 10.1038/s42003-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ekholm-Reed S., Goldberg M.S., Schlossmacher M.G., Reed S.I. Parkin-Dependent Degradation of the F-Box Protein Fbw7 Promotes Neuronal Survival in Response to Oxidative Stress by Stabilizing Mcl. Mol. Cell. Biol. 2013;33:3627–3643. doi: 10.1128/MCB.00535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berthet A., Bezard E., Porras G., Fasano S., Barroso-Chinea P., Dehay B., Martinez A., Thiolat M.L., Nosten-Bertrand M., Giros B., et al. L-DOPA impairs proteasome activity in parkinsonism through D 1 dopamine receptor. J. Neurosci. 2012;32:681–691. doi: 10.1523/JNEUROSCI.1541-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim K.Y., Stevens M.V., Akter M.H., Rusk S.E., Huang R.J., Cohen A., Noguchi A., Springer D., Bocharov A.V., Eggerman T.L., et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Investig. 2011;121:3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kitada T., Pisani A., Karouani M., Haburcak M., Martella G., Tscherter A., Platania P., Wu B., Pothos E.N., Shen J. Impaired dopamine release and synaptic plasticity in the striatum of Parkin-/- mice. J. Neurochem. 2009;110:613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- 112.Frank-Cannon T.C., Tran T., Ruhn K.A., Martinez T.N., Hong J., Marvin M., Hartley M., Treviño I., O’Brien D.E., Casey B., et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. Mitochondrial Dysfunction and Oxidative Damage in parkin-deficient Mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 114.Goldberg M.S., Fleming S.M., Palacino J.J., Cepeda C., Lam H.A., Bhatnagar A., Meloni E.G., Wu N., Ackerson L.C., Klapstein G.J., et al. Parkin-deficient Mice Exhibit Nigrostriatal Deficits but not Loss of Dopaminergic Neurons. J. Biol. Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 115.Song L., McMackin M., Nguyen A., Cortopassi G. Parkin deficiency accelerates consequences of mitochondrial DNA deletions and Parkinsonism. Neurobiol. Dis. 2017;100:30–38. doi: 10.1016/j.nbd.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 116.Sliter D.A., Martinez J., Hao L., Chen X., Sun N., Fischer T.D., Burman J.L., Li Y., Zhang Z., Narendra D.P., et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–262. doi: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matheoud D., Sugiura A., Bellemare-Pelletier A., Laplante A., Rondeau C., Chemali M., Fazel A., Bergeron J.J., Trudeau L.-E., Burelle Y., et al. Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell. 2016;166:314–327. doi: 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 118.Ashrafi G., Schlehe J.S., LaVoie M.J., Schwarz T.L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rogers R.S., Tungtur S., Tanaka T., Nadeau L.L., Badawi Y., Wang H., Ni H.M., Ding W.X., Nishimune H. Impaired mitophagy plays a role in denervation of neuromuscular junctions in ALS mice. Front. Neurosci. 2017;11:1–17. doi: 10.3389/fnins.2017.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madeo G., Martella G., Schirinzi T., Ponterio G., Shen J., Bonsi P., Pisani A. Aberrant striatal synaptic plasticity in monogenic parkinsonisms. Neuroscience. 2012;211:126–135. doi: 10.1016/j.neuroscience.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 121.Chen L., Xie Z., Turkson S., Zhuang X. A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration. J. Neurosci. 2015;35:890–905. doi: 10.1523/JNEUROSCI.0089-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pearlstein E., Michel F.J., Save L., Ferrari D.C., Hammond C. Abnormal development of glutamatergic synapses afferent to dopaminergic neurons of the pink1–/– mouse model of Parkinson’s disease. Front. Cell. Neurosci. 2016;10:1–13. doi: 10.3389/fncel.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kubli D.A., Cortez M.Q., Moyzis A.G., Najor R.H., Lee Y., Gustafsson Å.B. PINK1 is dispensable for mitochondrial recruitment of parkin and activation of mitophagy in cardiac myocytes. PLoS ONE. 2015;10:e0130707. doi: 10.1371/journal.pone.0130707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gispert S., Ricciardi F., Kurz A., Azizov M., Hoepken H.H., Becker D., Voos W., Leuner K., Müller W.E., Kudin A.P., et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS ONE. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pellegrini L., Hauser D.N., Li Y., Mamais A., Beilina A., Kumaran R., Wetzel A., Nixon-Abell J., Heaton G., Rudenko I., et al. Proteomic analysis reveals co-ordinated alterations in protein synthesis and degradation pathways in LRRK2 knockout mice. Hum. Mol. Genet. 2018;27:3257–3271. doi: 10.1093/hmg/ddy232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parisiadou L., Yu J., Sgobio C., Xie C., Liu G., Sun L., Gu X.-L., Lin X., Crowley N.A., Lovinger D.M., et al. LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat. Neurosci. 2014;17:367–376. doi: 10.1038/nn.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cho H.J., Yu J., Xie C., Rudrabhatla P., Chen X., Wu J., Parisiadou L., Liu G., Sun L., Ma B., et al. Leucine-rich repeat kinase 2 regulates Sec16A at ER exit sites to allow ER –Golgi export. EMBO J. 2014;33:2314–2331. doi: 10.15252/embj.201487807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sepulveda B., Mesias R., Li X., Yue Z., Benson D.L. Short- and Long-Term Effects of LRRK2 on Axon and Dendrite Growth. PLoS ONE. 2013;8:e61986. doi: 10.1371/journal.pone.0061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dwyer Z., Rudyk C., Thompson A., Farmer K., Fenner B., Fortin T., Derksen A., Sun H., Hayley S. Leucine-rich repeat kinase-2 (LRRK2) modulates microglial phenotype and dopaminergic neurodegeneration. Neurobiol. Aging. 2020;91:45–55. doi: 10.1016/j.neurobiolaging.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 130.Maekawa T., Tsushima H., Kawakami F., Kawashima R., Kodo M., Imai M., Ichikawa T. Leucine-rich repeat kinase 2 is associated with activation of the paraventricular nucleus of the hypothalamus and stress-related gastrointestinal dysmotility. Front. Neurosci. 2019;13:1–10. doi: 10.3389/fnins.2019.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang H., Zhang X., Zuo Z., Zhang Q., Pan Y., Zeng B., Li W., Wei H., Liu Z. Rip2 Is Required for Nod2-Mediated Lysozyme Sorting in Paneth Cells. J. Immunol. 2017;198:3729–3736. doi: 10.4049/jimmunol.1601583. [DOI] [PubMed] [Google Scholar]
- 132.Ferrazza R., Cogo S., Melrose H., Bubacco L., Greggio E., Guella G., Civiero L., Plotegher N. LRRK2 deficiency impacts ceramide metabolism in brain. Biochem. Biophys. Res. Commun. 2016;478:1141–1146. doi: 10.1016/j.bbrc.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 133.Giaime E., Tong Y., Wagner L.K., Yuan Y., Huang G., Shen J. Age-Dependent Dopaminergic Neurodegeneration and Impairment of the Autophagy-Lysosomal Pathway in LRRK-Deficient Mice. Neuron. 2017;96:796–807.e6. doi: 10.1016/j.neuron.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong Y., Yamaguchi H., Giaime E., Boyle S., Kopan R., Kelleher R.J., Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of α-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. USA. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xing W., Liu J., Cheng S., Vogel P., Mohan S., Brommage R. Targeted disruption of leucine-rich repeat kinase 1 but not leucine-rich repeat kinase 2 in mice causes severe osteopetrosis. J. Bone Miner. Res. 2013;28:1962–1974. doi: 10.1002/jbmr.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takagawa T., Kitani A., Fuss I., Levine B., Brant S.R., Tajima M., Nakamura S., Strober W. Dectin-1–Induced immunity in a mouse model of colitis. Sci. Transl. Med. 2019;10:1–25. doi: 10.1126/scitranslmed.aan8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beccano-kelly D.A., Volta M., Munsie L.N., Paschall S.A. LRRK2 overexpression alters glutamatergic presynaptic plasticity, striatal dopamine tone, postsynaptic signal transduction, behavioural hypoactivity and memory deficits. Hum. Mol. Genet. 2014;24:1336–1349. doi: 10.1093/hmg/ddu543. [DOI] [PubMed] [Google Scholar]
- 138.Parisiadou L., Xie C., Hyun J.C., Lin X., Gu X.L., Long C.X., Lobbestael E., Baekelandt V., Taymans J.M., Sun L., et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Henry A.G., Aghamohammadzadeh S., Samaroo H., Chen Y., Mou K., Needle E., Hirst W.D. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- 140.Beccano-Kelly D.A., Kuhlmann N., Tatarnikov I., Volta M., Munsie L.N., Chou P., Cao L.P., Han H., Tapia L., Farrer M.J., et al. Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Front. Cell. Neurosci. 2014;8:1–11. doi: 10.3389/fncel.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park J., Lee J.-W., Cooper S.C., Broxmeyer H.E., Cannon J.R., Kim C.H. Parkinson disease–associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 response. J. Leukoc. Biol. 2017;102:1093–1102. doi: 10.1189/jlb.1A0417-147RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daher J.P.L., Abdelmotilib H.A., Hu X., Volpicelli-Daley L.A., Moehle M.S., Fraser K.B., Needle E., Chen Y., Steyn S.J., Galatsis P., et al. Leucine-rich repeat kinase 2 (LRRK2) pharmacological inhibition abates α-synuclein gene-induced neurodegeneration. J. Biol. Chem. 2015;290:19433–19444. doi: 10.1074/jbc.M115.660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith N.J., Fuller M., Saville J.T., Cox T.M. Reduced cerebral vascularization in experimental neuronopathic Gaucher disease. J. Pathol. 2018;244:120–128. doi: 10.1002/path.4992. [DOI] [PubMed] [Google Scholar]
- 144.Yamada T., Dawson T.M., Yanagawa T., Iijima M., Sesaki H. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy. Autophagy. 2019;15:2012–2018. doi: 10.1080/15548627.2019.1643185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kelm-Nelson C.A., Brauer A.F.L., Barth K.J., Lake J.M., Sinnen M.L.K., Stehula F.J., Muslu C., Marongiu R., Kaplitt M.G., Ciucci M.R. Characterization of early-onset motor deficits in the Pink1−/− mouse model of Parkinson disease. Brain Res. 2018;1680:1–12. doi: 10.1016/j.brainres.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Das Banerjee T., Dagda R.Y., Dagda M., Chu C.T., Rice M., Vazquez-Mayorga E., Dagda R.K. PINK1 regulates mitochondrial trafficking in dendrites of cortical neurons through mitochondrial PKA. J. Neurochem. 2017;142:545–559. doi: 10.1111/jnc.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haque M.E., Mount M.P., Safarpour F., Abdel-Messih E., Callaghan S., Mazerolle C., Kitada T., Slack R.S., Wallace V., Shen J., et al. Inactivation of Pink1 gene in Vivo sensitizes dopamine-producing neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and can be rescued by autosomal recessive Parkinson disease genes, Parkin or DJ-1. J. Biol. Chem. 2012;287:23162–23170. doi: 10.1074/jbc.M112.346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martella G., Madeo G., Schirinzi T., Tassone A., Sciamanna G., Spadoni F., Stefani A., Shen J., Pisani A., Bonsi P. Altered profile and D2-dopamine receptor modulation of high voltage-activated calcium current in striatal medium spiny neurons from animal models of Parkinson’s disease. Neuroscience. 2011;177:240–251. doi: 10.1016/j.neuroscience.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 149.Billia F., Hauck L., Konecny F., Rao V., Shen J., Mak T.W. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc. Natl. Acad. Sci. USA. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kitada T., Pisani A., Porter D.R., Yamaguchi H., Tscherter A., Martella G., Bonsi P., Zhang C., Pothos E.N., Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl. Acad. Sci. USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matheoud D., Cannon T., Voisin A., Penttinen A.M., Ramet L., Fahmy A.M., Ducrot C., Laplante A., Bourque M.J., Zhu L., et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1 −/− mice. Nature. 2019;571:565–569. doi: 10.1038/s41586-019-1405-y. [DOI] [PubMed] [Google Scholar]
- 152.Moisoi N., Fedele V., Edwards J., Martins L.M. Loss of PINK1 enhances neurodegeneration in a mouse model of Parkinson’s disease triggered by mitochondrial stress. Neuropharmacology. 2014;77:350–357. doi: 10.1016/j.neuropharm.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martella G., Platania P., Vita D., Sciamanna G., Cuomo D., Tassone A., Tscherter A., Kitada T., Bonsi P., Shen J., et al. Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin. Exp. Neurol. 2009;215:388–396. doi: 10.1016/j.expneurol.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bhatia D., Chung K.P., Nakahira K., Patino E., Rice M.C., Torres L.K., Muthukumar T., Choi A.M.K., Akchurin O.M., Choi M.E. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight. 2019;4:1–20. doi: 10.1172/jci.insight.132826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Auburger G., Gispert S., Brehm N. Methyl-arginine profile of brain from aged PINK1-KO+A53T-SNCA mice suggests altered mitochondrial biogenesis. Parkinsons. Dis. 2016;2016:4686185. doi: 10.1155/2016/4686185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cabin D.E., Gispert-Sanchez S., Murphy D., Auburger G., Myers R.R., Nussbaum R.L. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol. Aging. 2005;26:25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 157.Gispert S., Brehm N., Weil J., Seidel K., Rüb U., Kern B., Walter M., Roeper J., Auburger G. Potentiation of neurotoxicity in double-mutant mice with Pink1 ablation and A53T-SNCA overexpression. Hum. Mol. Genet. 2015;24:1061–1076. doi: 10.1093/hmg/ddu520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jin G., Xu C., Zhang X., Long J., Rezaeian A.H., Liu C., Furth M.E., Kridel S., Pasche B., Bian X.-W., et al. Atad3a suppresses Pink1-dependent mitophagy to maintain homeostasis of hematopoietic progenitor cells. Nat. Immunol. 2018;19:29–40. doi: 10.1038/s41590-017-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang R., Tan J., Chen T., Han H., Tian R., Tan Y., Wu Y., Cui J., Chen F., Li J., et al. ATP13A2 facilitates HDAC6 recruitment to lysosome to promote autophagosome–lysosome fusion. J. Cell Biol. 2019;218:267–284. doi: 10.1083/jcb.201804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rayaprolu S., Seven Y.B., Howard J., Duffy C., Altshuler M., Moloney C., Giasson B.I., Lewis J. Partial loss of ATP13A2 causes selective gliosis independent of robust lipofuscinosis. Mol. Cell. Neurosci. 2018;92:17–26. doi: 10.1016/j.mcn.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 161.Schultheis P.J., Fleming S.M., Clippinger A.K., Lewis J., Tsunemi T., Giasson B., Dickson D.W., Mazzulli J.R., Bardgett M.E., Haik K.L., et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited α-synuclein accumulation and age-dependent sensorimotor deficits. Hum. Mol. Genet. 2013;22:2067–2082. doi: 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burchell V.S., Nelson D.E., Sanchez-Martinez A., Delgado-Camprubi M., Ivatt R.M., Pogson J.H., Randle S.J., Wray S., Lewis P.A., Houlden H., et al. The Parkinson’s disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat. Neurosci. 2013;16:1257–1265. doi: 10.1038/nn.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Braschi E., Goyon V., Zunino R., Mohanty A., Xu L., McBride H.M. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 2010;20:1310–1315. doi: 10.1016/j.cub.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 164.Outeiro T.F., Harvey K., Dominguez-Meijide A., Gerhardt E. LRRK2, alpha-synuclein, and tau: Partners in crime or unfortunate bystanders? Biochem. Soc. Trans. 2019;47:827–838. doi: 10.1042/BST20180466. [DOI] [PubMed] [Google Scholar]
- 165.Cresto N., Gardier C., Gubinelli F., Gaillard M.C., Liot G., West A.B., Brouillet E. The unlikely partnership between LRRK2 and α-synuclein in Parkinson’s disease. Eur. J. Neurosci. 2019;49:339–363. doi: 10.1111/ejn.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Toyofuku T., Okamoto Y., Ishikawa T., Sasawatari S., Kumanogoh A. LRRK 2 regulates endoplasmic reticulum–mitochondrial tethering through the PERK -mediated ubiquitination pathway. EMBO J. 2020;39:e100875. doi: 10.15252/embj.2018100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ekstrand M.I., Galter D. The MitoPark Mouse—An animal model of Parkinson’s disease with impaired respiratory chain function in dopamine neurons. Parkinsonism Relat. Disord. 2009;15(Suppl. 3):S185–S188. doi: 10.1016/S1353-8020(09)70811-9. [DOI] [PubMed] [Google Scholar]
- 168.Zhang S., Wang R., Wang G. Impact of Dopamine Oxidation on Dopaminergic Neurodegeneration. ACS Chem. Neurosci. 2019;10:945–953. doi: 10.1021/acschemneuro.8b00454. [DOI] [PubMed] [Google Scholar]
- 169.Muñoz P., Huenchuguala S., Paris I., Segura-Aguilar J. Dopamine Oxidation and Autophagy. Parkinsons. Dis. 2012;2012:920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kanďár R. The ratio of oxidized and reduced forms of selected antioxidants as a possible marker of oxidative stress in humans. Biomed. Chromatogr. 2016;30:13–28. doi: 10.1002/bmc.3529. [DOI] [PubMed] [Google Scholar]
- 171.Nagase M., Yamamoto Y., Matsumoto N., Arai Y., Hirose N. Increased oxidative stress and coenzyme Q10 deficiency in centenarians. J. Clin. Biochem. Nutr. 2018;63:129–136. doi: 10.3164/jcbn.17.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sourris K.C., Harcourt B.E., Tang P.H., Morley A.L., Huynh K., Penfold S.A., Coughlan M.T., Cooper M.E., Nguyen T.-V., Ritchie R.H., et al. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic. Biol. Med. 2012;52:716–723. doi: 10.1016/j.freeradbiomed.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 173.Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A., et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15:558–564. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- 174.Melser S., Zottola A.C.P., Serrat R., Puente N., Grandes P., Marsicano G., Hebert-Chatelain E. Functional Analysis of Mitochondrial CB1 Cannabinoid Receptors (mtCB1) in the Brain. Methods Enzymol. 2017;593:143–174. doi: 10.1016/bs.mie.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 175.Valenzuela R., Costa-Besada M.A., Iglesias-Gonzalez J., Perez-Costas E., Villar-Cheda B., Garrido-Gil P., Melendez-Ferro M., Soto-Otero R., Lanciego J.L., Henrion D., et al. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016;7:e2427. doi: 10.1038/cddis.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lindsay J., Laurin D., Verreault R., Hébert R., Helliwell B., Hill G.B., McDowell I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 177.Espinosa J., Rocha A., Nunes F., Costa M.S., Schein V., Kazlauckas V., Kalinine E., Souza D.O., Cunha R.A., Porciúncula L.O. Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J. Alzheimer’s Dis. JAD. 2013;34:509–518. doi: 10.3233/JAD-111982. [DOI] [PubMed] [Google Scholar]
- 178.Cunha R.A. Cafeína, receptores de adenosina, memoria y enfermedad de Alzheimer. Med. Clin. 2008;131:790–795. doi: 10.1016/S0025-7753(08)75506-4. [DOI] [PubMed] [Google Scholar]
- 179.Canas P.M., Porciuncula L.O., Cunha G.M.A., Silva C.G., Machado N.J., Oliveira J.M.A., Oliveira C.R., Cunha R.A. Adenosine A2A Receptor Blockade Prevents Synaptotoxicity and Memory Dysfunction Caused by -Amyloid Peptides via p38 Mitogen-Activated Protein Kinase Pathway. J. Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Eskelinen M.H., Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimer’s Dis. 2010;20:S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 181.Eskelinen M.H., Ngandu T., Tuomilehto J., Soininen H., Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimer’s Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 182.Pedata F., Pugliese A.M., Melani A., Gianfriddo M. A2A receptors in neuroprotection of dopaminergic neurons. Neurology. 2003;61:S49–S50. doi: 10.1212/01.WNL.0000095212.19029.04. [DOI] [PubMed] [Google Scholar]
- 183.Franco R., Navarro G. Adenosine A2A receptor antagonists in neurodegenerative diseases: Huge potential and huge challenges. Front. Psychiatry. 2018;9:1–5. doi: 10.3389/fpsyt.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Franco R. Café y salud mental. Aten. Primaria. 2009;41:578–581. doi: 10.1016/j.aprim.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abbas M.M., Xu Z., Tan L.C.S. Epidemiology of Parkinson’s Disease-East Versus West. Mov. Disord. Clin. Pract. 2018;5:14–28. doi: 10.1002/mdc3.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 187.Ribeiro J.A., Sebastiao A.M. Caffeine and adenosine. J. Alzheimer’s Dis. 2010;20:S3–S15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 188.Qi H., Li S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson’s disease. Geriatr. Gerontol. Int. 2014;14:430–439. doi: 10.1111/ggi.12123. [DOI] [PubMed] [Google Scholar]
- 189.Liu R., Guo X., Park Y., Huang X., Sinha R., Freedman N.D., Hollenbeck A.R., Blair A., Chen H. Caffeine intake, smoking, and risk of parkinson disease in men and women. Am. J. Epidemiol. 2012;175:1200–1207. doi: 10.1093/aje/kwr451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.