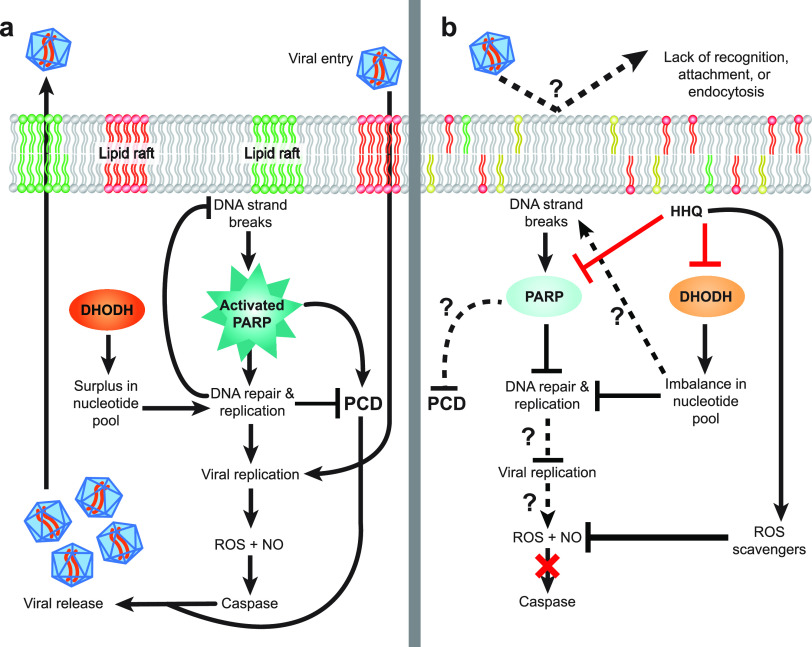
FIG 6.
Proposed model for the role of HHQ in influencing viral success in E. huxleyi. (a) During the infection of a phytoplankton cell, viruses are recognized via specific surface receptors and will enter the cell via endocytosis through distinct lipid rafts. Once inside the cell, the virus hijacks host replication machinery to produce additional viral particles. This replication is dependent on functional de novo nucleotide synthesis enzymes, such as dihydroorotate dehydrogenase (DHODH), to provide the cell with sufficient nucleotide materials. Likewise, functional DNA repair, often mediated by poly(ADP-ribose) polymerase (PARP), is necessary to ensure that replication can continue. Successful viral replication then generates intracellular reactive oxygen species (ROS) and nitric oxide (NO) signaling, which in turn activates caspase proteases, allowing the release of replicated viral particles via programmed cell death (PCD)-induced cell lysis. (b) In HHQ-exposed phytoplankton cells, virus-induced mortality was not observed, but the mechanism by which HHQ impacts viral cycling remains unclear. HHQ may directly inhibit (shown as red lines) the activity of DHODH and PARP, which would prevent the production of viral particles via the collapse of DNA replication machinery. HHQ may also indirectly impact parts of the virus cycle (shown as dotted lines) by changing host physiology to disrupt recognition, nucleotide production, ROS production, caspase activation, or PCD.