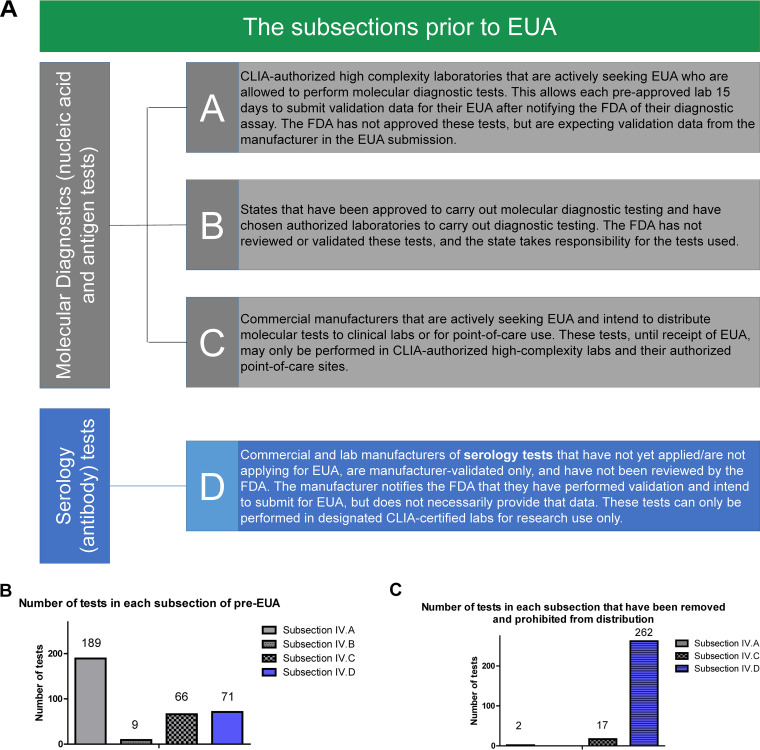
FIG 2.
The subsections of pre-FDA Emergency Use Authorization. (A) Manufacturers can market antibody tests through several pathways, described in the FDA Policy on Diagnostic Tests. The FDA stated that tests could be used without submission for EUA in order to encourage test development. No tests within these subsections have received FDA EUA, or FDA approval. As of 11 May 2020, Subsections IV.A through IV.C refer only to molecular/antigen-based diagnostic tests. Subsection IV.D refers only to serology tests (both lab developed and commercial). (B) The number of tests under each subsection at the time of publication. (C) The number of tests under each subsection that have been removed and prohibited from distribution at the time of publication. Sources for data are included in the References.