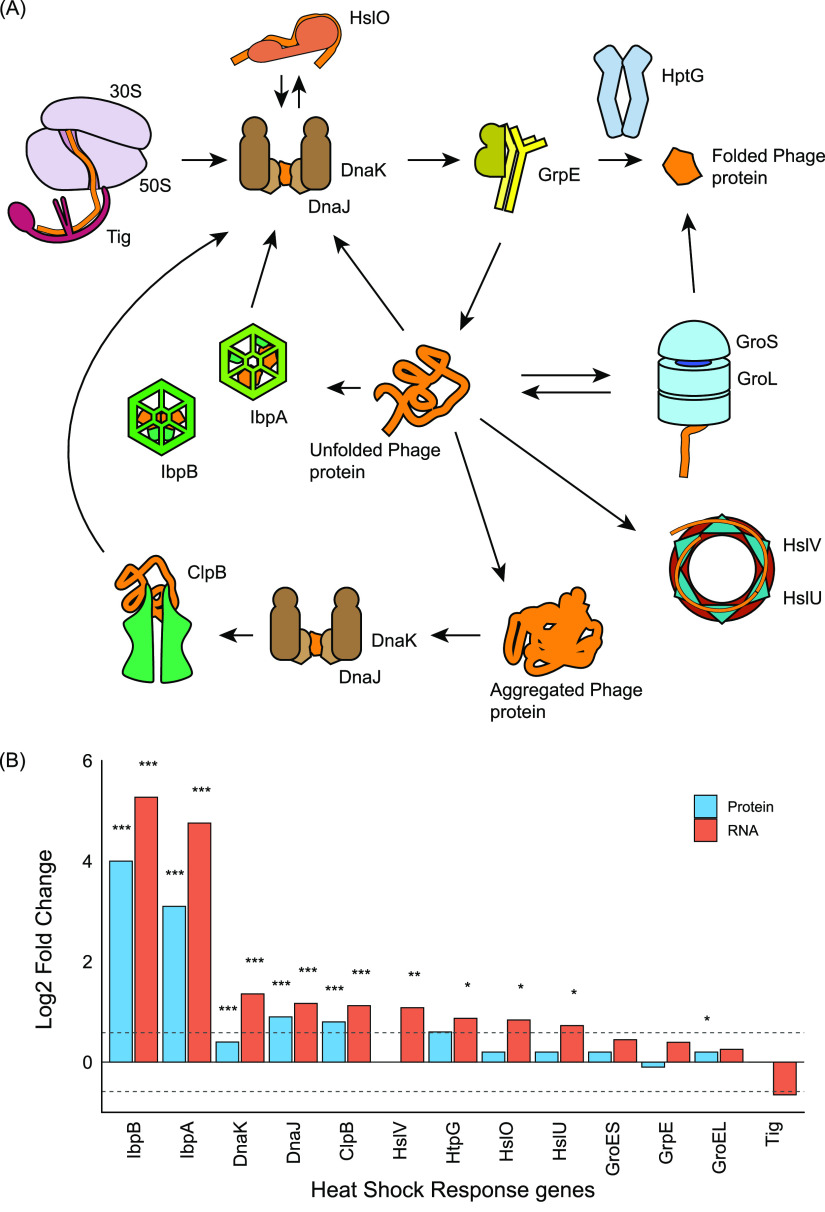
FIG 7.
Large changes to gene expression across the heat shock chaperone and protein folding pathway in E. coli C122 during φX174 infection. Proteins depicted in this pathway are transcriptionally regulated by transcription factor RpoH (σ32), which we saw upregulated during infection. (A) The chaperone system revealing the process controlling protein folding from synthesis to native protein state. The chaperone trigger factor (Tig) binds to the translating ribosome and the growing peptide chain. The DnaK/J chaperone proteins aid in folding by binding to hydrophobic regions of nascent proteins, preventing their misfolding and aggregation. DnaK/J may bind to HtpG to reactivate inactive protein substrates on the path to native protein fold (93). The holdase HslO binds to unfolded protein, which is released to DnaK for folding (94). GrpE may interact with DnaK, thereby dissociating ADP and releasing the bound protein (95). Small heat shock chaperones IbpA/B bind nascent proteins, preventing their irreversible aggregation into inclusion bodies, and await the availability of folding chaperones DnaK/J and GroEL/ES. The main folding machine of the system is the GroEL/ES complex. This ATP-dependent system facilitates folding of nascent proteins within the complex’s inner surface, releasing proteins in their native state. In the case of protein aggregates produced from improperly or partially folded proteins, the chaperone ClpB mediates their disaggregation for their refolding. Unfolded protein may also shuffle to the HslUV protease/ATPase complex for proteolysis (96). (B) Differential expression of transcripts and proteins was measured at 60 and 75 min, respectively. Dashed lines indicate the log2 fold change ± 0.58 values used as significance criteria. P values are shown as *** for ≤0.001, ** for ≤0.01, and * for ≤0.05.