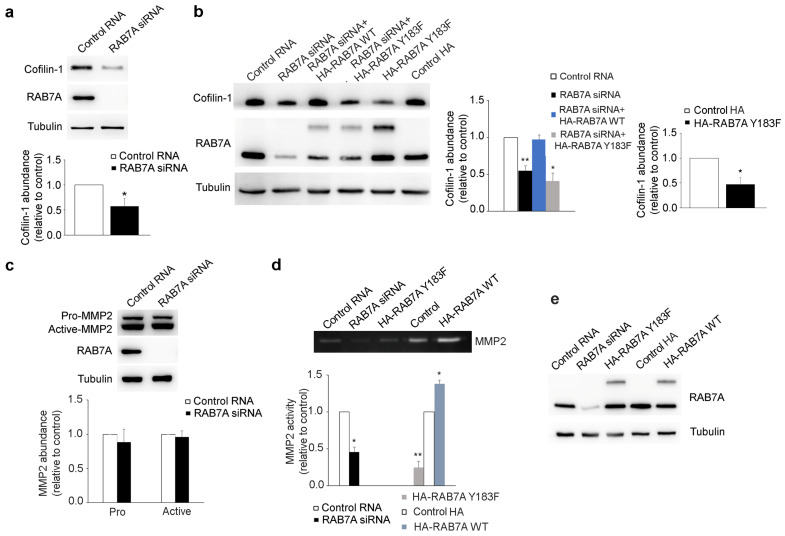
Figure 7.
RAB7A regulates cofilin-1 abundance and matrix metalloproteinase 2 (MMP2) activity. (a) HeLa cells were transfected with either control RNA or RAB7A siRNA, replated 72 h after transfection and then lysed after 48 h. Lysates were subjected to Western blot analysis using the indicated antibodies. Quantification of cofilin-1 abundance is shown. (b) HeLa cells were treated with either control RNA or RAB7A siRNA or transfected with plasmids coding for HA or HA-RAB7AY183F. HeLa cells silenced for RAB7A were also transfected with plasmids encoding HA-RAB7AWT or Y183F to rescue RAB7A functions. Lysates were subjected to Western blot analysis using the indicated antibodies. Quantification of cofilin-1 abundance is shown. (c) HeLa cells were transfected with either control RNA or RAB7A siRNA, replated 72 h after transfection and then lysed after 48 h. Lysates were subjected to Western blot analysis using anti-MMP2, anti-tubulin, and anti-RAB7A antibodies. Quantifications of pro-MMP2 and active-MMP2 abundance are shown. (d) gelatin zymography was performed using conditioned medium of HeLa cells treated with either control RNA or RAB7A siRNA or transfected with plasmids encoding HA, HA-RAB7AWT, or HA-RAB7AY183F. Quantification of MMP2 activity is shown. (e) HeLa cells, treated with either control RNA or RAB7A siRNA or transfected with plasmids encoding HA, HA-RAB7AWT, or HA-RAB7AY183F and whose conditioned medium was used for gelatin zymography, were lysed and lysates were subjected to Western blot analysis using anti-tubulin and anti-RAB7A antibodies. All data represent the mean ± s.e.m. of three independent experiments. * = p < 0.05; ** = p < 0.01.