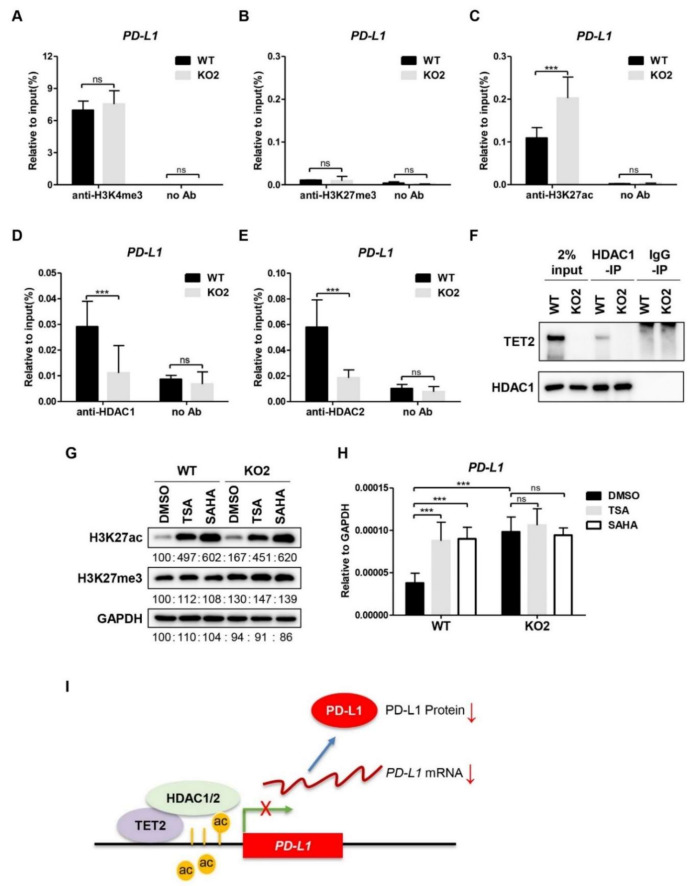
Figure 3.
TET2 recruits HDAC1/2 to deacetylate H3K27ac at PD-L1 promoter. (A) ChIP-qPCR analysis of H3K4me3 enrichment at PD-L1 promoter in WT and TET2 KO MCF7 cells. (B) ChIP-qPCR analysis of H3K27me3 enrichment at PD-L1 promoter in WT and TET2 KO MCF7 cells. (C) ChIP-qPCR analysis of H3K27ac enrichment at PD-L1 promoter in WT and TET2 KO MCF7 cells. (D,E) ChIP-qPCR analysis of the occupancy of HDAC1 and HDAC2 at the PD-L1 promoter in WT and TET2 KO MCF7 cells. (F) Western blot analysis of the anti-HDAC1-IP and IgG-IP products in WT and TET2 KO MCF7 cells. (G) Western blot analysis of the global H3K27ac levels in WT and TET2 KO MCF7 cells treated with or without HDAC inhibitors (TSA 1 μM; SAHA 5 μM). (H) RT-qPCR analysis of the relative mRNA expression levels of PD-L1 in WT and TET2 KO MCF7 cells treated with or without HDAC inhibitors (TSA 1 μM; SAHA 5 μM). (I) Schematic diagram of the working model in which TET2 inhibits PD-L1 gene transcription through HDAC1/2-mediated histone deacetylation. (***, p < 0.001; ns, not significant.)