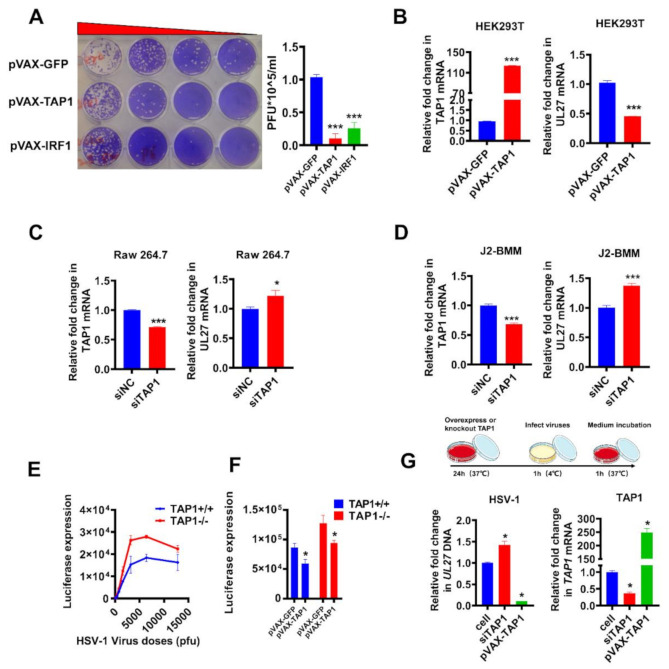
Figure 3.
Validation of viral inhibition by loss-of-function and gain-of-function experiments. (A)Vero cells were transfected with plasmids (1 μg/mL) expressing GFP, TAP1, and IRF1 for 24 h, respectively, followed by HSV-1 infection (MOI = 0.25) for 8 h. Then, the titer of HSV-1 in the supernatant was quantified by plaque assay. (B) HEK293T cells were transfected with plasmids (1 μg/mL) expressing GFP and TAP1 for 24 h, respectively, followed by HSV-1 infection (MOI = 0.25) for 8 h. Then, the expression of TAP1 and HSV-1 UL27 were quantified by RT-qPCR. (C,D) Raw 264.7 and WT-J2-BMM cells were transfected with small interfering RNA (siRNA) (siNC as negative control) for 24 h, followed by HSV-1 infection (MOI = 0.25) for 8 h. Then, the expression of TAP1 and HSV-1 UL27 were quantified by RT-qPCR. The expression level of mRNA was normalized to the expression of β-actin. (E) TAP1-wildtype (TAP1+/+) or TAP1-knockout (TAP1−/−) fibroblast cells were infected with HSV-1, and subsequently, the Renilla luciferase activity in cell lysates was determined at 8 h post infection. (F) TAP1+/+ or T TAP1−/− fibroblast cells were transfected with or without TAP1-expressing plasmid (1 μg/mL) for 24 h, followed by HSV-1 infection (MOI = 0.25) for 8 h, and then the Renilla luciferase activity in cell lysates was determined. (G) HEK293 cells were transfected with plasmids (1 μg/mL) expressing TAP1 or siTAP1 for 24 h, respectively, followed by HSV-1 incubation (MOI = 1) for 1 h at 4 °C. Then, aspirated and discarded the supernatant, washed with PBS three times, added culture medium, incubated at 37 °C for 1 h, and the expression of HSV-1 (UL27) and TAP1 were quantified by qPCR. The expression level was normalized to the expression of β-actin, and the data from at least triplicates were shown as the mean ± SD. * p < 0.05, and *** p < 0.001.