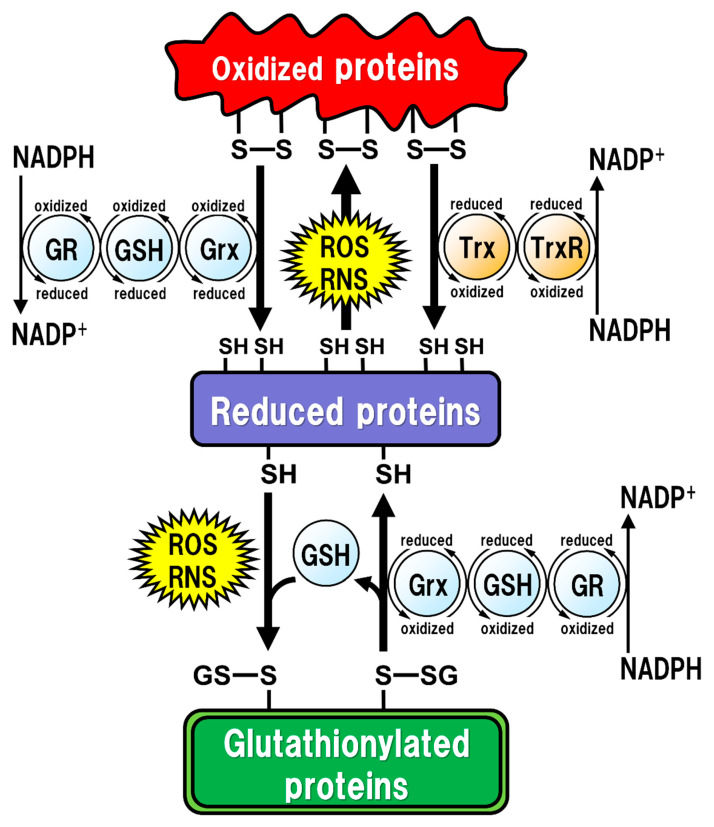
Figure 1.
Regulation of the intracellular protein redox state by glutathione (GSH), glutaredoxin (Grx), and thioredoxin (Trx). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) cause protein dysfunction, which is induced by the oxidation of thiol (SH) residues to form disulfide (S-S) bonds in the active site. Grx and Trx regulate protein function by reducing the S-S bonds of the substrate proteins. Consequently, Grx and Trx themselves result in the oxidized forms, which are reduced back by GSH and Trx reductase (TrxR), respectively. Oxidized GSH (GSSG) is reduced back to GSH by GSH reductase (GR). Both oxidized TrxR and GR are reduced by receiving electrons from nicotinamide adenine dinucleotide phosphate (NADPH). Under oxidative stress conditions, GSH can bind to cysteine residues (GS-S) in a process known as ‘S-glutathionylation’ to prevent the irreversible dysfunction of the proteins. Grx also functions in the deglutathionylation of the GS-S containing proteins to resume protein functions under physiological conditions.