Abstract
Honey has good antimicrobial properties and can be used for medical treatment. The antimicrobial properties of unifloral honey varieties are different. In this study, we evaluated the antimicrobial and antioxidant activities of nine kinds of Chinese monofloral honeys. In addition, headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) technology was used to detect their volatile components. The relevant results are as follows: 1. The agar diffusion test showed that the diameter of inhibition zone against Staphylococcus aureus of Fennel honey (21.50 ± 0.41 mm), Agastache honey (20.74 ± 0.37 mm), and Pomegranate honey (18.16 ± 0.11 mm) was larger than that of Manuka 12+ honey (14.27 ± 0.10 mm) and Manuka 20+ honey (16.52 ± 0.12 mm). The antimicrobial activity of Chinese honey depends on hydrogen peroxide. 2. The total antioxidant capacity of Fennel honey, Agastache honey, and Pomegranate honey was higher than that of other Chinese honeys. There was a significant positive correlation between the total antioxidant capacity and the total phenol content of Chinese honey (r = 0.958). The correlation coefficient between the chroma value of Chinese honey and the total antioxidant and the diameter of inhibition zone was 0.940 and 0.746, respectively. The analyzed dark honeys had better antimicrobial and antioxidant activities. 3. There were significant differences in volatile components among Fennel honey, Agastache honey, Pomegranate honey, and Manuka honey. Hexanal-D and Heptanol were the characteristic components of Fennel honey and Pomegranate honey, respectively. Ethyl 2-methylbutyrate and 3-methylpentanoic acids were the unique compounds of Agastache honey. The flavor fingerprints of the honey samples from different plants can be successfully built using HS-GC-IMS and principal component analysis (PCA) based on their volatile compounds. Fennel honey, Agastache honey, and Pomegranate honey are Chinese honey varieties with excellent antimicrobial properties, and have the potential to be developed into medical grade honey.
Keywords: Chinese honey, antimicrobial activity, Fennel honey, Agastache honey, Pomegranate honey, volatile profile
1. Introduction
Honey is a health care product with high nutritional value, and it is also a traditional medicine that prevents and treats wound infections [1]. In recent decades, the extensive use of antibiotics has brought about bacterial resistance, and the emergence of super bacteria has caused people concern, the call for returning to traditional antimicrobial drugs is increasing. As a representative of traditional antimicrobial remedies, honey has received more and more attention, and there is more and more research on its antimicrobial properties [2].
The botanical origin of honey is important because it can significantly affect the phytochemicals present and can consequently impact on the antimicrobial capacity [3]. Many in vitro and in vivo studies have demonstrated the antimicrobial, antifungal, and antiviral activity of honey [4,5,6]. The antimicrobial ability of honey is the result of a combination of many factors, which is also believed to be the reason that honey does not produce bacterial resistance. The high osmotic pressure and low pH of honey are not conducive to the growth of microorganisms. Honey still has antimicrobial properties after being diluted, indicating that it contains other antimicrobial active compounds. Hydrogen peroxide, methylglyoxal (MGO), and polyphenolic compounds were considered to be the key compounds for honey to exert antimicrobial activity [3].
Hydrogen peroxide is the main component of honey to exert peroxide antimicrobial activity, and it is also the first antimicrobial substance identified in honey [7]. Hydrogen peroxide is mainly produced by glucose oxidase incorporated into nectar by bees, and its function may be to prevent the fermentation of immature honey [8]. When mature honey is diluted to 30–50% of the original concentration, the hydrogen peroxide has the highest accumulation rate [1]. The content of hydrogen peroxide in honey is different, and hydrogen peroxide is the main antimicrobial component of most honey [9].
After neutralizing the hydrogen peroxide in honey, most honey will lose its antimicrobial activity. However, the antimicrobial activity of Manuka honey was not affected. The pronounced non-hydrogen peroxide antimicrobial activity of Manuka honey directly originated from MGO and its concentration in Manuka honey ranged from 38 mg/kg to 761 mg/kg, which was up to 100-fold higher compared to ordinary honeys [10]. The correlation between the content of MGO in Manuka honey and its non-hydrogen peroxide antimicrobial activity was as high as 0.98 [11]. The content of MGO in Manuka honey is an important basis for its grading.
Polyphenols, as secondary metabolites of plants, are the main compounds in honey that promote human health [12]. Polyphenols can scavenge free radicals and inhibit oxidation, and a variety of polyphenolic compounds in honey have been identified as having antimicrobial activity [13,14]. The polyphenols composition in honey mainly depends on its floral origin, and it can in fact also be used as a tool for authentication of the botanical origin of honey, especially in the case of unifloral varieties [15].
The aroma of honey is specific due to the combination of volatile compounds present in low concentrations. Honey volatiles can be used as a fingerprint for the botanical origin of honey [16]. Some of the aroma compounds exhibit antimicrobial properties. The volatile components in Hungarian honeys have antimicrobial effects, and their content is 0.12–0.26% [17]. Volatile and non-volatile/semi-volatile compounds may be contributed partly to the antimicrobial activity of Ulmo honey [18].
The antimicrobial and antioxidant capacity of honey has been widely confirmed. It has been developed into medical treatment in the form of medical grade honey. As a representative of special honey, Manuka honey has been recognized by consumers for its superior antimicrobial activity and is a world-renowned medical grade honey. China is a big beekeeping country and has a wealth of nectar plant species. The number of bee colonies and volume of honey production have long been ranked first in the world. Are there any honey varieties in China that have antimicrobial properties close to or exceeding Manuka honey and what is their antimicrobial mechanism? In order to solve this question, we researched nine species of unifloral Chinese honeys, hoping to find Chinese honey varieties with excellent antimicrobial ability.
2. Results and Discussion
2.1. Conventional Physical and Chemical Indexes
In order to ensure that the botanical origin of unifloral honey samples was authentic, we commissioned trusted beekeepers to sample at designated locations during the corresponding flowering period. The information of nine Chinese unifloral honey samples was shown in Table 1. We tested the physicochemical properties of the honey samples, and the relevant results are shown in Table 2. To further determine the purity of the samples, we measured the pollen ratio of the honey samples. The corresponding pollen percentages of Chinese monofloral honey samples were all greater than or equal to 75.00 ± 1.50%, which indicated that the purity of our monofloral honeys was high.
Table 1.
The information of 9 species of unifloral Chinese honey samples.
| Plant Sources (Latin Name) | Plant Sources (Common Name) | Producing Area | Sampling Time |
|---|---|---|---|
| Zanthoxylum bungeanum Maxim | Pepper | Zhejiang Province | 2015 |
| Punica granatum L. | Pomegranate | Yunnan Province | 2016 |
| Brassica napus L. | Rape | Jiangsu Province | 2016 |
| Eriobotrya japonica (Thunb.) Lindl | Loquat | Zhejiang Province | 2017 |
| Vitex negundo L. | Vitex | Shaanxi Province | 2017 |
| Crataegus pinnatifida Bunge | Hawthorn | Shaanxi Province | 2017 |
| Helianthus annuus L. | Sunflower | Shaanxi Province | 2017 |
| Agastache rugosa (Fisch. et Mey.) O. Ktze | Agastache | Yunnan Province | 2017 |
| Foeniculum vulgare Mill | Fennel | Gansu Province | 2017 |
Table 2.
Values of physicochemical parameters of the different varieties of honey.
| Honey | Pollen (%) | Water Content (%) | Diastase Activity (DN) | Chroma Value (mm) | HMF (mg/kg) | Protein (%) | Ash Content (%) | Fructose (%) | Glucose (%) |
|---|---|---|---|---|---|---|---|---|---|
| Manuka 20+ | 30.67 ± 0.81 | 17.00 ± 0.15 | 7.22 ± 0.18 | 150 ± 1.00 | 27.87 ± 0.35 | 0.31 ± 0.02 | 0.36 ± 0.03 | 40.12 ± 0.75 | 31.02 ± 1.35. |
| Manuka 12+ | 50.91 ± 1.84 | 16.84 ± 0.27 | 9.12 ± 0.08 | 150 ± 0.00 | 33.54 ± 0.14 | 0.29 ± 0.02 | 0.42 ± 0.02 | 36.06 ± 0.58 | 30.51 ± 1.12 |
| Fennel | 78.33 ± 0.86 | 20.56 ± 0.12 | 27.12 ± 0.10 | 82 ± 0.00 | 4.62 ± 0.07 | 0.83 ± 0.03 | 0.24 ± 0.01 | 38.14 ± 0.84 | 22.86 ± 0.13 |
| Agastache | 80.36 ± 1.27 | 20.68 ± 0.23 | 21.58 ± 0.07 | 71 ± 0.00 | 2.44 ± 0.10 | 0.35 ± 0.02 | 0.37 ± 0.01 | 34.66 ± 0.57 | 28.94 ± 0.16 |
| Pomegranate | 84.94 ± 1.10 | 17.88 ± 0.35 | 21.96 ± 0.19 | 43 ± 1.00 | 1.97 ± 0.08 | 0.31 ± 0.01 | 0.25 ± 0.03 | 36.49 ± 0.74 | 34.12 ± 0.50 |
| Hawthorn | 75.00 ± 1.50 | 20.56 ± 0.14 | 22.22 ± 0.08 | 66 ± 1.00 | 2.12 ± 0.03 | 0.47 ± 0.02 | 0.15 ± 0.01 | 34.47 ± 0.17 | 28.68 ± 0.28 |
| Pepper | 83.87 ± 1.19 | 20.04 ± 0.35 | 23.01 ± 0.12 | 44 ± 0.00 | 12.18 ± 0.03 | 0.33 ± 0.03 | 0.22 ± 0.01 | 35.55 ± 0.41 | 34.63 ± 0.23 |
| Sunflower | 85.26 ± 1.16 | 23.32 ± 0.09 | 12.70 ± 0.22 | 21 ± 1.00 | 1.02 ± 0.06 | 0.23 ± 0.03 | 0.16 ± 0.01 | 36.30 ± 0.43 | 33.92 ± 0.13 |
| Loquat | 93.94 ± 1.20 | 22.00 ± 0.26 | 13.93 ± 0.08 | 3 ± 0.00 | 1.11 ± 0.06 | 0.14 ± 0.01 | 0.06 ± 0.01 | 34.22 ± 0.30 | 29.36 ± 0.14 |
| Rape | 95.24 ± 1.53 | 18.76 ± 0.08 | 22.94 ± 0.05 | 24 ± 0.00 | 1.98 ± 0.04 | 0.31 ± 0.02 | 0.08 ± 0.01 | 37.78 ± 0.57 | 35.22 ± 0.29 |
| Vitex | 90.40 ± 1.21 | 21.16 ± 0.10 | 22.12 ± 0.07 | 11 ± 0.00 | 0.90 ± 0.07 | 0.30 ± 0.01 | 0.05 ± 0.01 | 39.28 ± 0.65 | 28.75 ± 0.22 |
Data represent the mean of triplicate readings ± standard deviations (SD).
The water contents for seven Chinese honeys were over 20%. This may be related to the production mode of honey in China. In order to pursue the yield or the purity of unifloral honey, many Chinese honeys do not have enough time to mature in beehives. The diastase activity of Manuka honey was significantly lower than that of Chinese honey. This may be related to the high dose of MGO in Manuka honey. MGO has negative effects on the structure and function of proteins in Manuka honey [19]. The values of HMF for Manuka honeys were quite high in comparison with the rest of the honeys. Manuka honeys were purchased from New Zealand Comvita Ltd. (Wellington, New Zealand), which had been stored at room temperature for a longer time before sampling than other samples. In addition, the possible heating treatment in the factory may have also increased the content of HMF in Manuka honeys. Chinese honey samples were all raw honey, which were directly stored in the refrigerator after being taken out from the hives.
The chroma value of Manuka honey 20+ was as high as 150 ± 1.00 mm. Fennel honey and Agastache honey have chroma values of 82 ± 0.00 mm and 71 ± 0.00 mm respectively, which were significantly higher than other Chinese honeys. The chroma value was positively correlated with total antioxidant capacity and inhibition zone diameter. The correlation between the chroma value of Chinese honeys and the total antioxidant capacity was 0.940. The correlation between the chroma value with the inhibition zone diameter was 0.746. Dark honey contained a lot of antioxidant components, and the antimicrobial ability of dark honey was generally stronger than that of light honey [20,21]. Our research showed that darker honeys provide high levels of antioxidants as well as antimicrobial effectiveness.
2.2. Antimicrobial Test
The antimicrobial activity of honey samples against Staphylococcus aureus (gram-positive), Escherichia coli (gram-negative), and Candida albicans (fungus) was determined by the agar diffusion and broth macrodilution method. These three microorganisms were the recommended species for the antimicrobial test in the Chinese disinfection technical specifications. 10% phenol and Manuka honey were used as positive control. The negative control was distilled water. The agar-well diffusion test used with 50% solution of honey. All honey samples had no bacteriostatic ring against E. coli and C. albicans. As for S. aureus, nine Chinese honey samples had a bacteriostatic ring larger than 11 mm in diameter (Table 3). The difference in the sensitivity of gram-positive bacteria and gram-negative bacteria to honey is a result of the difference in the composition of their cell walls. Compared with gram-negative bacteria, gram-positive bacteria have no outer membrane to protect the peptidoglycan layer, which makes it easier for the antimicrobial agents to penetrate and cause damage [22]. Manuka honey has been proven to inhibit the gram-positive bacterium Enterococcus faecalis, while gram-negative E. coli was more resistant to it [23]. The biofilm of C. albicans is special and can switch from a yeast form to a filamentous form. This increases the difficulty of suppressing it [24]. The bacteriostatic ring diameter of Fennel honey (21.50 ± 0.41 mm), Agastache honey (20.74 ± 0.37 mm), and Pomegranate honey (18.16 ± 0.11 mm) against S. aureus was significantly larger than other honeys, including Manuka honey 12+ (14.27 ± 0.10 mm) and Manuka honey 20+ (16.52 ± 0.12 mm). The artificial honey sample had no bacteriostatic ring, indicating that the antimicrobial effect was not simply conditional to the sugar content in the honey. It is well known that the agar-well diffusion method is suitable for the detection of antimicrobial effect, but if the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) are to be calculated, a more sensitive method, such as broth dilution method, must be used. By this method, the microorganisms are in direct contact with the testing inhibiting substance. The antimicrobial effect does not depend on the diffusibility of the reagent through the agar medium [25].
Table 3.
The antimicrobial activity and main antimicrobial substance content of honey samples.
| Plant Sources | Inhibition Diameters (mm) | H2O2 (μm/g) | MGO (mg/kg) |
|---|---|---|---|
| Fennel | 21.50 ± 0.41 | 2882.76 ± 10.86 | - |
| Agastache | 20.74 ± 0.37 | 1161.14 ± 4.70 | - |
| Pomegranate | 18.16 ± 0.11 | 2150.89 ± 32.30 | - |
| Manuka 20+ | 16.52 ± 0.12 | 7.31 ± 0.72 | 268.27 ± 0.35 |
| Manuka12+ | 14.27 ± 0.10 | 92.26 ± 0.13 | 109.95 ± 0.23 |
| Vitex | 13.41 ± 0.25 | 2351.31 ± 5.43 | - |
| Pepper | 12.73 ± 0.49 | 428.15 ± 1.03 | - |
| Hawthorn | 12.69 ± 0.76 | 1579.83 ± 3.70 | - |
| Sunflower | 12.46 ± 0.98 | 144.24 ± 0.90 | - |
| Rape | 12.07 ± 0.17 | 1534.04 ± 3.20 | - |
| Loquat | 11.57 ± 0.27 | 966.82 ± 10.86 | - |
| Phenol | 31.39 ± 0.15 | - | - |
The data of inhibition zone were obtained from the agar diffusion test against S. aureus. Data represent the mean of triplicate readings ± standard deviations (SD)–means below LOD.
Broth micro-dilution method was used to detect MIC and MBC of the 11 honey samples with obvious bacteriostatic ring by agar-well diffusion test. 16 h, 12 h, and 18 h were the rapid growth periods of K/2 of S. aureus, E. coli, and C. albicans, respectively, which were determined as the determination time of MIC experiment. For each honey sample, ten concentration gradients were prepared for this test. We found that the inhibition rate was not linearly related to the concentration. The result of broth dilution assay (Table 4) showed that the inhibitory effect of honey on S. aureus was better than that of E. coli and C. albicans, which was consistent with the result of the agar-well diffusion test. The MIC of honey samples on E. coli were all significantly lower than that on C. albicans. This was consistent with the report that C. albicans was difficult to inhibit [24]. The MBC of honey samples was relatively high, close to 50%, which is related to the low concentration of bactericidal substances in honey. The antimicrobial ability of honey is the result of a combination of many compounds [1]. The MBC of Fennel honey (25.0–40.0%) against S. aureus was lower than that of other honeys, including Manuka honey (>50.0%). Fennel honey, Agastache honey, and Pomegranate honey were three kinds of Chinese honeys with excellent antimicrobial effect. The results of MIC and MBC also showed that honey has a better inhibitory effect on gram-positive bacteria.
Table 4.
Minimum inhibitory concentrations and minimum bactericidal concentrations of honey samples. The antimicrobial activity of honey was assessed against S. aureus, E. coli, and C. albicans.
| Honey | S. aureus | E. coli | C. albicans | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC90 | MIC50 | MBC | MIC90 | MIC50 | MBC | MIC90 | MIC50 | MBC | |
| Manuka 20+ | 2.5–3.1 | 1.4–2.5 | >50.0 | 3.1–5.0 | 3.1–5.0 | >50.0 | >50.0 | 25.0–50.0 | >50.0 |
| Manuka 12+ | 2.5–3.1 | 1.4–2.5 | >50.0 | 3.1–5.0 | 3.1–5.0 | >50.0 | >50.0 | 25.0–50.0 | >50.0 |
| Pomegranate | 2.5–3.1 | 2.5–3.1 | >50.0 | 12.5–20.0 | 12.5–20.0 | >50.0 | 25.0–50.0 | 25.0–50.0 | >50.0 |
| Fennel | 5.0–6.3 | 5.0–6.3 | 25.0–40.0 | >50.0 | 33.3–50.0 | 25.0–40.0 | >50.0 | 12.5–20.0 | 40.0–50.0 |
| Vitex | 6.3–10.0 | 6.3–10.0 | >50.0 | 12.5–20.0 | 12.5–20.0 | 25.0–50.0 | 25.0–50.0 | 20.0–25.0 | 25.0–50.0 |
| Hawthorn | 6.3–10.0 | 6.3–10.0 | >50.0 | 12.5–20.0 | 12.5–20.0 | 25.0–50.0 | 25.0–50.0 | 25.0–50.0 | >50.0 |
| Rape | 6.3–10.0 | 6.3–10.0 | >50.0 | 12.5–20.0 | 12.5–20.0 | 25.0–50.0 | 25.0–50.0 | 25.0–50.0 | >50.0 |
| Agastache | 10.0–12.5 | 5.0–6.3 | >50.0 | 12.5–20.0 | 10.0–12.5 | 25.0–50.0 | >50.0 | 25.0–50.0 | >50.0 |
| Sunflower | 10.0–12.5 | 6.3–10.0 | >50.0 | 12.5–20.0 | 12.5–20.0 | >50.0 | 25.0–50.0 | 25.0–50.0 | >50.0 |
| Pepper | 10.0–12.5 | 6.3–10.0 | >50.0 | 20.0–25.0 | 6.3–10.0 | >50.0 | 25.0–50.0 | 25.0–50.0 | >50.0 |
| Loquat | 20.0–25.0 | 20.0–25.0 | >50.0 | 25.0–40.0 | 12.5–20.0 | >50.0 | >50.0 | 25.0–50.0 | >50.0 |
MIC90: 90% minimum inhibitory concentration. MIC50: 50% minimum inhibitory concentration. The concentration was the percentage ratio of mass to volume (w/v, %).
2.3. Analysis of Antimicrobial Components in Honey
In order to study the antimicrobial mechanism of Chinese honey, we analyzed the antimicrobial components of the above 11 honey samples. Hydrogen peroxide is considered to be the main antimicrobial substance in honey [7]. After adding catalase, the antimicrobial effect of all nine Chinese honey samples was abolished, while the two Manuka honey samples still maintained a significant antimicrobial effect on S. aureus. MGO was identified as the main antimicrobial component of Manuka honey [26]. We tested the hydrogen peroxide and MGO content of the honey samples. Hydrogen peroxide concentrations ranged from 7.31 ± 0.72 µm/g to 2882.76 ± 10.86 µm/g in different honey samples (Table 3). The content of hydrogen peroxide in Chinese honey was significantly higher than that in Manuka honey. Fennel honey, Agastache honey, and Pomegranate honey produced 2882.76 ± 10.86 µm/g, 1161.14 ± 4.70 µm/g and 2150.89 ± 32.30 µm/g of hydrogen peroxide, whereas Manuka 12+ (92.26 ± 0.13 µm/g) and Manuka 20+ (7.31 ± 0.72 µm/g) produced low amounts of hydrogen peroxide. This result can be attributed to the observation that Manuka honey lacks hydrogen peroxide [5].
The MGO content of Manuka 12+ and 20+ was 109.95 ± 0.23 mg/kg and 268.27 ± 0.35 mg/kg, respectively, which is not significantly different from the content on the label. MGO was not detected (below LOD) in all nine Chinese honey samples. The artificial honey sample had no antimicrobial activity, which indicates that the antimicrobial effect is not simply conditional to the sugar content in the honey. Hydrogen peroxide may be the main antimicrobial factor of Chinese honey samples. The correlation coefficient between the hydrogen peroxide content and the diameter of the inhibition zone in Chinese honey was 0.535, indicating that its antimicrobial activity was the result of the combined action of multiple bacteriostatic components. These results clearly indicate that factors other than hydrogen peroxide production, for example, polyphenolic and volatile compounds, contribute to the antimicrobial activity. Therefore, we identified polyphenolic and volatile compounds in the honeys and their possible relation to the antimicrobial activity was assessed.
Polyphenolic compounds have been recognized as the main reason for the antioxidant activity of honey. Many polyphenolic compounds in honey have also been identified as having antimicrobial activity [3]. The content of total phenols and total flavonoids in honey and the antioxidant capacity of honey were measured (Table 5). The correlation of these indicators was analyzed (Table 6). The total phenol content of Fennel honey (81.27± 1.31 mg/100 g, GAE) was between Manuka 12+ (66.14 ± 0.92 mg/100 g, GAE) and Manuka 20+ (83.54 ± 0.23 mg/100 g, GAE), which was significantly higher than that of other Chinese honeys. Alzahrani [20] reported that Manuka honey had the highest phenolic content (899.09 ± 11.75 mg/kg, GAE) among the four floral honeys examined in their research. Our research results were essentially the same. The total antioxidant capacity of Fennel honey (2601.84 ± 51.23 μg/100 g, Rutin) was higher than other Chinese honeys. Pomegranate honey (48.88 ± 0.27 mg/100 g, QE) has the highest total flavonoid content in Chinese honeys. The DPPH scavenging ability of Agastache honey (0.77 ± 0.02 mg/mL) was the best among the Chinese honeys, between Manuka honey 12 + (0.69 ± 0.02 mg/mL) and Manuka honey 20 + (0.80 ± 0.03 mg/mL).
Table 5.
The antioxidant activity, total phenols, and total flavonoids content of tested honeys.
| Honey | Total Phenols | Total Flavonoids | Radical Scavenging Activity (DPPH) | Total Antioxidant Capacity |
|---|---|---|---|---|
| (mg/100 g, GAE) | (mg/100 g, QE) | (IC50, mg/mL) | (μg/100 g, rutin) | |
| Manuka 20+ | 83.54 ± 0.23 | 60.72 ± 0.29 | 0.80 ± 0.03 | 4224.17 ± 21.39 |
| Manuka 12+ | 66.14 ± 0.92 | 50.15 ± 0.12 | 0.69 ± 0.02 | 3091.97 ± 27.66 |
| Fennel | 81.27± 1.31 | 31.81 ± 0.08 | 0.23 ± 0.18 | 2601.84 ± 51.23 |
| Agastache | 50.72 ± 0.12 | 37.33 ± 0.04 | 0.77 ± 0.02 | 2136.31 ± 18.94 |
| Hawthorn | 50.28 ± 0.42 | 32.16 ± 0.22 | 0.32 ± 0.03 | 1660.72 ± 22.31 |
| Pomegranate | 39.35 ± 0.31 | 48.88 ± 0.27 | 0.66 ± 0.02 | 1271.72 ± 3.84 |
| Pepper | 37.18 ± 0.23 | 28.22 ± 0.04 | 0.22 ± 0.02 | 1140.29 ± 13.61 |
| Sunflower | 19.72 ± 0.53 | 26.44 ± 0.07 | 0.21 ± 0.01 | 1018.65 ± 6.47 |
| Rape | 20.17 ± 0.23 | 21.22 ± 0.04 | 0.20 ± 0.01 | 985.20 ± 14.31 |
| Vitex | 21.46 ± 0.31 | 22.30 ± 0.07 | 0.12 ± 0.02 | 935.05 ± 6.60 |
| Loquat | 12.91 ± 0.12 | 21.40 ± 0.31 | 0.17 ± 0.03 | 687.61 ± 4.99 |
Data represent the mean of triplicate readings ± standard deviations (SD). GAE: gallic acid. QE: quercetin.
Table 6.
Correlation analysis between indicators of Chinese honeys.
| Total Phenols | Total Flavonoids | Radical Scavenging Activity | Total Antioxidant Capacity | Inhibition Diameters | H2O2 | Chroma Value | |
|---|---|---|---|---|---|---|---|
| Total phenols | 1.000 | ||||||
| Total flavonoids | 0.509 | 1.000 | |||||
| Radical scavenging activity | 0.342 | 0.846 ** | 1.000 | ||||
| Total antioxidant capacity | 0.958 *** | 0.462 | 0.428 | 1.000 | |||
| Inhibition diameters | 0.795 ** | 0.666 | 0.653 | 0.853 ** | 1.000 | ||
| H2O2 | 0.525 | 0.263 | 0.065 | 0.466 | 0.535 | 1.000 | |
| Chroma value | 0.948 *** | 0.577 | 0.507 | 0.940 *** | 0.746 * | 0.337 | 1.000 |
* means p < 0.05. ** means p < 0.01. *** means p < 0.001.
Phenolics derived from nectar are the main antioxidants of honey. Plant species have a significant impact on the antioxidant capacity of honey [27]. Many reports have shown a significant correlation between the total phenolic content and total antioxidant activity of honey [20,28]. In our research, there was a significant positive correlation between total phenolic content and total antioxidant capacity of Chinese honey (r = 0.958). The total phenol content of Chinese honey was positively correlated with the diameter of inhibition zone (r = 0.795). The phenolic compounds in honey may play a certain antimicrobial effect. Flavone content was positively correlated with DPPH scavenging power (r = 0.846). The correlation between total antioxidant capacity and inhibition zone diameter of Chinese honey was 0.853. The antimicrobial ability of honey can be predicted by its total antioxidant capacity.
2.4. Volatile Compounds in Honey
Some aroma compounds exhibit antimicrobial properties [17,18]. Volatile compounds have great importance in characterizing honey’s botanical source, which directly influences their sensory characteristics [16]. Headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS), with no complex pretreatment and high sensitivity, has been widely used in food volatile components analysis [29].
We detected the volatile components of the three kinds of honey (Fennel honey, Agastache honey, and Pomegranate honey), and their antimicrobial properties were higher than that of Manuka honey. The pollen percentage of Manuka 12+ honey (50.91 ± 1.84%) was higher than that of Manuka 20+ honey (30.67 ± 0.81%). Therefore, Manuka 12+ honey was used as the control sample for volatile components detection. The volatile compounds in Fennel honey, Agastache honey, Pomegranate honey, and Manuka 12+ honey samples were isolated by HS and identified by GC-IMS. A total of 81 volatile components were detected, and 60 of them were identified (Table 7). Many volatiles identified in our study were aromatic compounds. Volatile benzene derivatives in honey were considered to have antimicrobial effects [30]. Volatile compounds potentially partly contributed to the antimicrobial activity of honey.
Table 7.
HS-GC-IMS integration parameters of volatile compounds in tested honey samples.
| Count | Compound | CAS # | Formula | MW | RI | Rt (sec) | Dt (a.u.) | Comment |
|---|---|---|---|---|---|---|---|---|
| 1 | Citral | C5392405 | C10H16O | 152.2 | 1482.3 | 1033.197 | 1.19082 | |
| 2 | 2,3-diethyl-5-methylpyrazine | C18138040 | C9H14N2 | 150.2 | 1164.3 | 580.939 | 1.27668 | |
| 3 | Cis-3,7-dimethyl-octa-2,6-dien-1-ol | C624157 | C10H18O | 154.3 | 1237.4 | 684.886 | 1.21783 | |
| 4 | Citronellal-M | C106230 | C10H18O | 154.3 | 1159.6 | 574.247 | 1.34073 | Monomer |
| 5 | 2-Ethyl-3,5-dimethylpyrazine | C13925070 | C8H12N2 | 136.2 | 1111.3 | 505.545 | 1.21609 | |
| 6 | Methyl octanoate-D | C111115 | C9H18O2 | 158.2 | 1110.1 | 503.76 | 1.94657 | Dimer |
| 7 | Citronellal-D | C106230 | C10H18O | 154.3 | 1157.2 | 570.715 | 1.84501 | Dimer |
| 8 | Linalool oxide-M | C60047178 | C10H18O2 | 170.3 | 1069.3 | 445.773 | 1.25933 | Monomer |
| 9 | Linalool oxide-D | C60047178 | C10H18O2 | 170.3 | 1068.3 | 444.363 | 1.81632 | Dimer |
| 10 | Trans-linalool oxide-M | C34995772 | C10H18O2 | 170.3 | 1090.1 | 475.387 | 1.26101 | Monomer |
| 11 | Trans-linalool oxide-D | C34995772 | C10H18O2 | 170.3 | 1085.8 | 469.182 | 1.81801 | Dimer |
| 12 | 1-phenylethanol-M | C98862 | C8H8O | 120.2 | 1060.4 | 433.081 | 1.18844 | Monomer |
| 13 | 1-phenylethanol-D | C98862 | C8H8O | 120.2 | 1060.6 | 433.363 | 1.56989 | Dimer |
| 14 | (Z)-2-octenal-M | C20664464 | C8H14O | 126.2 | 1050 | 418.281 | 1.32378 | Monomer |
| 15 | (Z)-2-octenal-D | C20664464 | C8H14O | 126.2 | 1046.1 | 412.758 | 1.73993 | Dimer |
| 16 | (E)-3-Octen-2-one-M | C18402829 | C8H14O | 126.2 | 1027.8 | 386.771 | 1.32197 | Monomer |
| 17 | (E)-3-Octen-2-one-D | C18402829 | C8H14O | 126.2 | 1029 | 388.395 | 1.73811 | Dimer |
| 18 | Benzene acetaldehyde-M | C122781 | C8H8O | 120.2 | 1040.6 | 404.962 | 1.25837 | Monomer |
| 19 | Octanal-M | C124130 | C8H16O | 128.2 | 1005.5 | 354.937 | 1.41465 | Monomer |
| 20 | Benzaldehyde-M | C100527 | C7H6O | 106.1 | 958.7 | 310.758 | 1.14933 | Monomer |
| 21 | Benzaldehyde-D | C100527 | C7H6O | 106.1 | 959.5 | 311.412 | 1.47185 | Dimer |
| 22 | (E,E)-2,4-Octadienal-M | C30361285 | C8H12O | 124.2 | 1124.2 | 523.896 | 1.26822 | Monomer |
| 23 | (E,E)-2,4-Octadienal-D | C30361285 | C8H12O | 124.2 | 1123 | 522.193 | 1.77926 | Dimer |
| 24 | Benzene acetaldehyde-D | C122781 | C8H8O | 120.2 | 1040 | 404.118 | 1.54104 | Dimer |
| 25 | Heptanol | C53535334 | C7H16O | 116.2 | 976.8 | 325.815 | 1.39978 | |
| 26 | Methyl octanoate-M | C111115 | C9H18O2 | 158.2 | 1109.8 | 503.387 | 1.48176 | Monomer |
| 27 | 3-Methylpentanoic acid | C105431 | C6H12O2 | 116.2 | 958.6 | 310.64 | 1.59685 | |
| 28 | Oct-1-en-3-ol | C3391864 | C8H16O | 128.2 | 983.6 | 331.468 | 1.15686 | |
| 29 | Octanal-D | C124130 | C8H16O | 128.2 | 1005.8 | 355.465 | 1.82448 | Dimer |
| 30 | 2-Acetylfuran-M | C1192627 | C6H6O2 | 110.1 | 911.8 | 271.782 | 1.11613 | Monomer |
| 31 | Heptanal-M | C111717 | C7H14O | 114.2 | 900.1 | 262.034 | 1.33289 | Monomer |
| 32 | Heptanal-D | C111717 | C7H14O | 114.2 | 900.9 | 262.644 | 1.69957 | Dimer |
| 33 | Cyclohexanone-M | C108941 | C6H10O | 98.1 | 894.8 | 257.566 | 1.15362 | Monomer |
| 34 | Cyclohexanone-D | C108941 | C6H10O | 98.1 | 894.8 | 257.566 | 1.45511 | Dimer |
| 35 | Furfural-M | C98011 | C5H4O2 | 96.1 | 829.2 | 221.417 | 1.08191 | Monomer |
| 36 | Furfural-D | C98011 | C5H4O2 | 96.1 | 828 | 220.808 | 1.33289 | Dimer |
| 37 | Hexanal-M | C66251 | C6H12O | 100.2 | 793 | 201.921 | 1.26118 | Monomer |
| 38 | Hexanal-D | C66251 | C6H12O | 100.2 | 793 | 201.921 | 1.56105 | Dimer |
| 39 | 2,3-Butanediol | C513859 | C4H10O2 | 90.1 | 781.5 | 196.082 | 1.35718 | |
| 40 | (Z)-2-Heptenal-M | C57266861 | C7H12O | 112.2 | 945.6 | 299.871 | 1.21477 | Monomer |
| 41 | (Z)-2-Heptenal-D | C57266861 | C7H12O | 112.2 | 946.8 | 300.885 | 1.67467 | Dimer |
| 42 | 3-methylbutan-1-ol | C123513 | C5H12O | 88.1 | 733.1 | 176.855 | 1.49745 | |
| 43 | 3-hydroxybutan-2-one | C513860 | C4H8O2 | 88.1 | 710.5 | 167.878 | 1.33205 | |
| 44 | Methyl butyrate | C623427 | C5H10O2 | 102.1 | 694.5 | 161.557 | 1.43472 | |
| 45 | 3-methylbutanal | C590863 | C5H10O | 86.1 | 647.9 | 147.532 | 1.1958 | |
| 46 | 2-methyl-1-propanol | C78831 | C4H10O | 74.1 | 620.7 | 139.725 | 1.17089 | |
| 47 | Butanal | C123728 | C4H8O | 72.1 | 556.7 | 121.362 | 1.27974 | |
| 48 | 2-Butanone | C78933 | C4H8O | 72.1 | 586.5 | 129.893 | 1.24377 | |
| 49 | 1-propene-3-methylthio | C10152768 | C4H8S | 88.2 | 682.2 | 157.364 | 1.0399 | |
| 50 | Ethyl Acetate | C141786 | C4H8O2 | 88.1 | 604.6 | 135.098 | 1.33325 | |
| 51 | 2-methylbutan-1-ol | C137326 | C5H12O | 88.1 | 747.7 | 182.666 | 1.47347 | |
| 52 | 2-methyl-2-butenal | C1115113 | C5H8O | 84.1 | 744.4 | 181.365 | 1.42826 | |
| 53 | 5-methylfurfural | C620020 | C6H6O2 | 110.1 | 967.1 | 317.751 | 1.1267 | |
| 54 | 2-heptanone | C110430 | C7H14O | 114.2 | 890.9 | 254.717 | 1.25824 | |
| 55 | Ethyl 2-methylbutyrate | C7452791 | C7H14O2 | 130.2 | 843.4 | 229.121 | 1.65051 | |
| 56 | 2-Acetylfuran-D | C1192627 | C6H6O2 | 110.1 | 912.8 | 272.586 | 1.44357 | Dimer |
| 57 | Ethyl phenylacetate | C101973 | C10H12O2 | 164.2 | 1239.1 | 687.359 | 1.30522 | |
| 58 | 3-Methyl-3-buten-1-ol | C763326 | C5H10O | 86.1 | 723.7 | 173.145 | 1.24758 | |
| 59 | γ-Octalactone | C104507 | C8H14O2 | 142.2 | 1279 | 744.06 | 1.34069 | |
| 60 | Citronellol | C106229 | C10H20O | 156.3 | 1238.6 | 686.629 | 1.36257 |
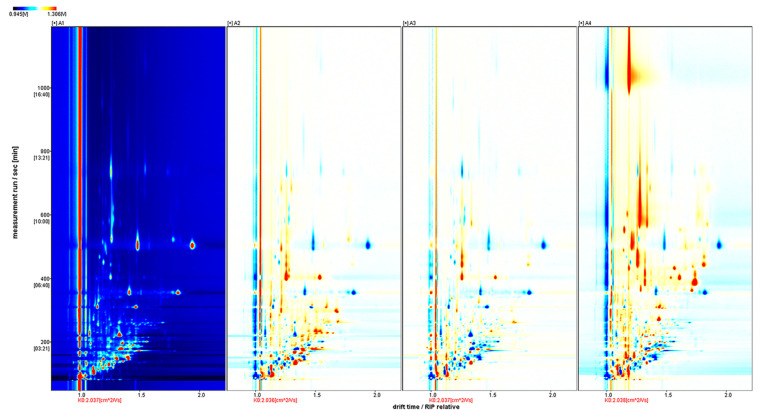
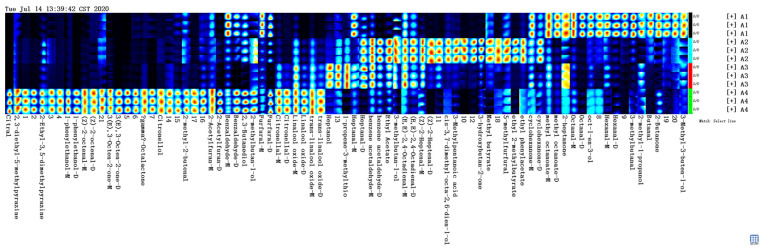
The two-dimensional (2D) array full-size top view plot (Figure 1) of HS-GC-IMS was obtained by the normalization of the ion migration time and reactive ion peak (RIP) position. The ion signal was described by different colors. Taking Fennel honey as a reference, red means high and blue means low. Increasing color darkness indicates increasing content. We used the Gallery Plot plug-in to draw the fingerprint spectrum of the volatile compounds of honey samples (Figure 2).
Figure 1.
Imaging of volatile compounds represented by HS-GC-IMS of honey samples. A1: Fennel honey; A2: Agastache honey; A3: Pomegranate honey; A4: Manuka 12+ honey.
Figure 2.
Gallery plot of the selected signal peak areas obtained with 4 kinds of honey samples. A1: Fennel honey; A2: Agastache honey; A3: Pomegranate honey; A4: Manuka 12+ honey.
There were significant differences in the composition and content of volatile components among honey samples. Manuka honey had the most volatile components. Citral, 1-phenylethanol, (Z)-2-octenal, (E)-3-Octen-2-one, γ-Octalactone, and Citronellol could be used as the characteristic components of Manuka honey, which was different from the other three kinds of honey. Hexanal-D was the characteristic component of Fennel honey. 3-Methylpentanoic acid and ethyl 2-methylbutyrate were the characteristic components of Agastache honey. Pomegranate honey had the least variety of flavors, and heptanol was its unique compound. Among the four kinds of honey, the volatile compounds of dark honey were more than that of light-colored honey. Dark honey seemed to have more volatile components and this may be related to its stronger antimicrobial activity.
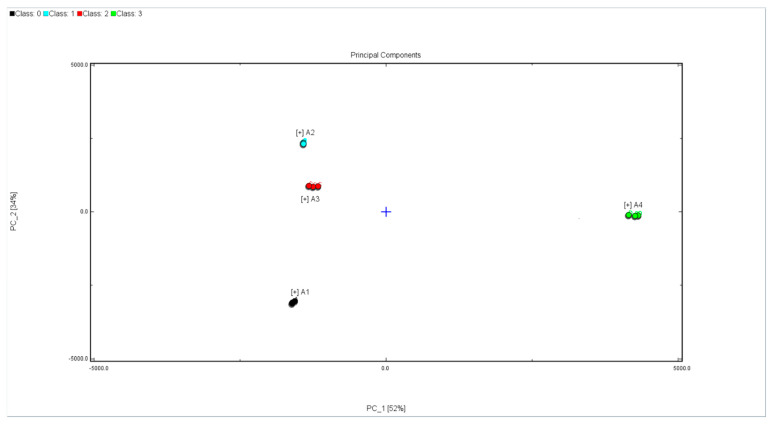
HS-GC-IMS applied with chemometrics, such as PCA (principal component analysis), could cluster honey with different floral origins. PCA could highlight the differences between samples. The PCA results of the flavor compounds in the four honeys are shown in Figure 3. Principal component 1 (PC1) expressed 52% of the variance and Principal component 2 (PC2) explained 34% of the variance. The loadings of each compound on the PCA explicitly showed that the grouping of the different unifloral honeys was mainly influenced by certain volatile compounds. PC1 and PC2 of all the samples explained 86% of the total variance at length. The PCA results indicated that the four samples occupied relatively independent spaces in the distribution map. The four kinds of honeys could be completely distinguished. HS-GC-IMS imaging coupled with PCA was a useful strategy to discriminate honey from different floral origins.
Figure 3.
PCA scores plot of preprocessed HS-GC-IMS spectra of Fennel honey, Agastache honey, Pomegranate honey, and Manuka 12+ honey. A1: Fennel honey; A2: Agastache honey; A3: Pomegranate honey; A4: Manuka 12+ honey.
The botanical origin is the major factor that determines the physicochemical properties of honey, due to the wide variability in the chemical structure of plant nectars and secretions. Geographical origins, climatic conditions, honeybee associated factors, and honey’s associated factors will also affect the quality of honey to a certain extent [31]. Further testing is merited to use a statistically significant number of honeys from a certain single botanical origin, place of production, and extraction date.
3. Materials and Methods
3.1. Honey Samples
In order to ensure that the botanical origin of unifloral honey samples was authentic, we commissioned trusted beekeepers to sample at designated locations during the corresponding flowering period. Nine Chinese unifloral honey samples were collected (Table 1). Manuka honey 12+ and 20+ were purchased from New Zealand Comvita Ltd. (Wellington, New Zealand). The collection time of all the samples was in 2015–2017. All honey samples were stored in a refrigerator at −20 °C away from light until analyzed.
3.2. Main Reagents and Equipment
3.2.1. Main Reagents
S. aureus (CICC 23926), E. coli (CICC 10899) and C. albicans (CICC 32380) were purchased from China Center of Industrial Culture Collection (Beijing, China). Catalase, methylglyoxal (HPLC grade), gallic acid, quercetin, and DPPH were purchased from Sigma-Aldrich (St. Louis, MO, USA). H2O2 quantitative analysis kit (water-compatible), LB broth, and phenol were purchased from Sangon Biotech (Shanghai, China). Nutrient agar was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
3.2.2. Main Equipments
Multiskan Sky: Thermo Scientific, Waltham, MA, USA. Shimadzu spectrophotometer UV-2550: Shimadzu Co., Ltd., Kyoto, Japan. Agilent 1200 liquid chromatograph: Agilent Technologies, Palo Alto, CA, USA. Agilent 490 gas chromatograph: Agilent Technologies, Palo Alto, CA, USA. IMS instrument: FlavourSpec®, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany.
3.3. Physicochemical Analysis
The water content was determined by an Abbe refractometer (MASTER-20M, ATAGO, Tokyo, Japan). The color intensity was determined using a Pfund honey colorimeter (HI96785, HANNA, Winsockit, RI, USA). The chroma value of solid honey was determined after complete decrystallization at 50 °C Ash content was obtained by placing honey (6 g) in a crucible and heating at 550 °C overnight in a muffle furnace. The protein content was determined by Bradford method [32]. The sugars in honey samples were carried out by an HPLC (Agilent 1200) with an ELSD-detector, according to the method described by Deng [28]. The hydroxymethylfurfural (HMF) content of honey samples was determined by the method reported by Ribeiro [33]. Diastase activity was determined using the method reported by Pasias [34].
The pollen detection in honey was carried out according to the method described by the PRC national standard (GB/T 23194-2008) [35] with minor modifications. 10 g honey was added to 20 mL 40 °C distilled water. After fully dissolved, it was centrifuged at 3000× g for 10 min. We discarded 4/5 of the supernatant, then added 2 mL of acetic acid, mixed well, and let stand for 2 h. Centrifuged at 3000× g for 10 min. Discarded 1/2 of the supernatant. Added 3 mL of freshly prepared mixture of acetic anhydride and sulfuric acid (9:1, volume ratio). After mixing, the sample was bathed at 90 °C for 7 min. Centrifuged at 3000× g for 10 min and then discarded the supernatant. The precipitate was washed 3 times with distilled water. Centrifuged after each cleaning. The final precipitate was pollen grains. The extracted pollen grains were stored in 1 mL 50% glycerol. We took a drop of sample solution on the blood cell counting plate. We let it stand for 5 min, and observed with a microscope. Pollen plant source identification refers to the pollen morphology of Chinese plants [36]. The predominant specific pollen was counted from the entire slide or until at least 400 pollens were counted. Three parallel tests were performed on each sample.
3.4. Antimicrobial Activity of Honey
Three bacterial strains were used for antimicrobial assays, including S. aureus (Gram-positive), E. coli (Gram-negative), and C. albicans (fungus), which are 3 common types of bacteria in clinic. These 3 microorganisms were also the recommended species for the antimicrobial test in the Chinese disinfection technical specifications. In order to determine the non-hydrogen peroxide antimicrobial activity of honey, catalase was added to honey samples to remove hydrogen peroxide. Corresponding to the amount of sugar in honey, an artificial honey sample was made by diluting 10.37 g fructose, 10.00 g glucose, and 0.38 g sucrose in 4.25 mL sterile Milli-Q water. Two different methods were used to evaluate the antimicrobial activity of honey: agar-well diffusion and broth micro-dilution. All experiments were performed in triplicate.
3.4.1. Agar Well Diffusion Assay
Suspensions of the bacteria were diluted in LB broth to provide a concentration of 107 CFU/mL. 100 µL of this suspension was added to 30 mL of agar at 50 °C and poured into a 9 cm diameter plate. After cooling, 4 holes with a diameter of 8 mm were drilled into the plate. The agar hole was sealed with a flame at the bottom. Each well was filled with 100 µL honey solution, and then the plates were incubated at 37 °C for 18 h. The diameter of the inhibition zones was measured by vernier caliper. Throughout the agar well diffusion experiments, a solution of 50% (w/v) honey was used, unless otherwise stated.
3.4.2. Broth Micro-Dilution Assay
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the honey samples were determined by the broth dilution method in 96-well microplates. We diluted the honey sample with LB broth. The final concentrations of diluted honey samples were 50%, 25%, 20%, 12.5%, 10%, 6.25%, 5%, 3.125%, 2.5%, 1.25%. The bacterial cultures were diluted to a final concentration of 106 CFU/mL. Tested honey solutions and bacterial suspension were mixed in a ratio of nine to one. 200 µL mixed solution was dispensed into each well of the 96-well microplate. The 96-well microplates were incubated at 37 °C. S. aureus was cultured for 16 h, E. coli for 12 h and C. albicans for 18 h. These times were the K/2 rapid growth periods of the three bacteria. The absorbance was measured at 600 nm. The minimum concentration of honey to inhibit bacterial growth was considered to be MIC. 50 µL bacteria suspension without bacteria growth in MIC test was cultured on nutrient agar plate and incubated at 37 °C for 24 h. The minimum concentration without bacterial growth was MBC.
3.5. Hydrogen Peroxide Assay
Honeys were diluted to 50% and the hydrogen peroxide content was measured as described in the protocol of H2O2 quantitative analysis kit (water-compatible). This kit (Item No: C500069) was purchased from Sangon Biotech (Shanghai, China). In an acidic environment, H2O2 can oxidize Fe2+ to Fe3+. Fe3+ ions combine with xylenol orange molecules to form Fe3+-xylenol orange complexes. The complexes have a maximum absorption wavelength of 560 nm or 595 nm, and the absorption value is proportional to the concentration of H2O2. The standard curve was made by H2O2 with a known concentration gradient (1–100 μM). Experiments were performed 3 times in duplicate.
3.6. Determination of Methylglyoxal Concentration in Honeys
The content of methylglyoxal was analyzed according to the method of Oelschlaegel [37] with minor modifications. The processed samples were analyzed by Agilent 1200 HPLC system. The analytical column was a Kromasil reversed phase chromatographic C18 column (4.6 mm × 150 mm, 5.0 μm). The mobile phase A was 0.1% acetic acid and mobile phase B was methanol. The elution conditions were: 0–5 min, 30% B; 5–10 min, 30% B–90% B; 10–15 min, 90% B; 15–16 min, 90% B–30% B; 16–20 min, 30% B. The flow rate was 1.0 mL/min.
3.7. Antioxidant Test
3.7.1. Total Phenolics
The total phenolics were determined by using the Folin–Ciocalteu method [38]. The honey sample (5 g) was diluted to 50 mL with distilled water and filtered through Whatman No. 1 paper. 1 mL of this solution was mixed with 1 mL Folin–Ciocalteu reagent and then mixed by vortexing. The mixed solution was treated with 5 mL of 1 M Na2CO3 solution, and then made up to 10 mL. The mixture was incubated at ambient temperature in the dark for 1.5 h. Absorbance was measured at 760 nm against an ethanol blank, and gallic acid was used as standard.
3.7.2. Total Flavonoids
Honey (5 g) was diluted to 25 mL with distilled water and filtered through Whatman No. 1 paper. 1mL of this solution was mixed with 0.3 mL of 15% NaNO3 solution, we shook it well and let stand for 6 min. We added 0.3 mL 10% Al(NO3)3 solution, shook well and let stand for 6 min, added 4 mL of 4% NaOH solution, diluted with 50% ethanol to 10 mL, shook well and let stand for 15 min. The absorbance was measured at 510 nm, and quercetin was used as standard.
3.7.3. DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of honey samples was determined according to the method of Scherer [39] with minor modifications. 2.7 mL DPPH solution in methanol (0.06 mM) was mixed with 0.3 mL of 50% (w/v) honey solution. The mixture was thoroughly mixed by vortexing and we let stand for 1.5 h in the dark. Then, the absorbance was measured at 517 nm.
3.7.4. Total Antioxidant Capacity
The total antioxidant capacity of honeys was determined according to the protocol of Atmani [40], with modifications. Honey (5 g) was diluted to 25 mL with distilled water and filtered through Whatman No. 1 paper. 1 mL of this solution was added to 2.5 mL of PBS (0.2 M, pH 6.6) and 2.5 mL of 1% K3Fe(CN)6 solution. This was incubated at 50 °C for 20 min. 2.5 mL 10% trichloroacetic acid was added to the mixture. Centrifuged at 5000× g for 5 min after standing for 10 min. We took 2.5 mL supernatant, added 2.5 mL distilled water, and 0.5 mL 0.1% FeCl3, shook well and let stand for 10 min. The absorbance was determined at 700 nm. The results were converted according to Rutin’s standard curve.
3.8. HS-GC-IMS
An Agilent 490 gas chromatograph and IMS instrument equipped with an automatic sampling device were used to detect the volatile substances in honey samples. The HS-GC-IMS analysis was performed as previously described [41] with minor modifications. Briefly, honey (2 g) was transferred into a 20 mL glass vial sealed with a silicon septum and magnetic metal crimp. The headspace bottle was incubated at 55 °C for 20 min. Then, 500 µL aliquots was automatically injected into the heated injector under splitless injection mode at 85 °C. The GC equipped with an FS-SE-54-CB-0.5 (15 m × 0.53 mm ID) column was used for separation at 60 °C. N2 (purity ≥ 99.99%) was used as the carried gas. The following were programmed as flows: initial flow of 2 mL/min, maintained 2 min, flow ramp up to 100 mL/min in 18 min, and maintained for 10 min. The drift tube length was 50 mm, which operated at a constant voltage of 400 V/cm. The drift tube was maintained at 45 °C under N2 as drift gas at 150 mL/min. The IMS cell was operated in the positive ion mode using helium as ionization source. Each spectrum had 32 scans with a grid pulse width of 100 μs, a repetition rate of 21 ms, and a sampling frequency of 150 kHz. PCA was used to analyze the differences in volatile components between samples of honey from 4 different plant sources, with mean-centered, UV scaled, and log-transformed data, before building the PCA model. LAV software version 2.2.1 (Gesellschaft fur Analytische Sensorsysteme mbH, Dortmund, Germany) was used to analyze the data.
3.9. Statistical Analysis
All analyses were carried out in triplicate, and the data were expressed as mean standard deviation (SD). Correlation analysis was achieved using SPSS 24.0 software (IBM SPSS Statistics; Chicago, IL, USA).
4. Conclusions
We researched the chemical composition and antimicrobial activity of nine unifloral Chinese honeys. The agar diffusion test and broth micro-dilution assay showed that Fennel honey, Agastache honey, and Pomegranate honey were Chinese honey varieties with excellent antimicrobial activity, compared to Manuka honeys. The antimicrobial activity of Chinese honey depends on hydrogen peroxide. There was a significant positive correlation between the total antioxidant capacity and the total phenol content of Chinese honey (r = 0.958). The correlation coefficient between the chroma value of Chinese honey and the total antioxidant and the diameter of inhibition zone was 0.940 and 0.746, respectively. The analyzed dark honeys had better antimicrobial and antioxidant activities. The flavor fingerprints of the honey samples could be successfully built using HS-GC-IMS and PCA, based on their volatile compounds. Hexanal-D and Heptanol were the characteristic components of Fennel honey and Pomegranate honey, respectively. Ethyl 2-methylbutyrate and 3-methylpentanoic acid were the unique compounds of Agastache honey. Fennel honey, Agastache honey, and Pomegranate honey have the potential to be developed into medical grade honey and deserve further study.
Author Contributions
Conceptualization, Y.-Z.Z., J.-J.S. and F.-L.H.; Formal analysis, G.-Z.Z.; Funding acquisition, F.-L.H.; Investigation, Y.-Z.Z. and J.-J.S.; Software, J.-J.S. and S.-S.L.; Supervision, F.-L.H.; Validation, H.-Q.Z.; Writing—original draft, Y.-Z.Z.; Writing—review & editing, S.-S.L., G.-Z.Z., S.W., H.-Q.Z. and F.-L.H. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the earmarked fund for Modern Agro-Industry Technology Research System from the Ministry of Agriculture of China (CARS-44).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no competing financial interest.
Sample Availability
Sample of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albaridi N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019;2019:1–10. doi: 10.1155/2019/2464507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLoone P., Warnock M., Fyfe L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immunol. 2016;49:161–167. doi: 10.1016/j.jmii.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Nolan V.C., Harrison J., Cox J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics. 2019;8:251. doi: 10.3390/antibiotics8040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain K.S., Hossain M.G., Moni A., Rahman M.M., Rahman U.H., Alam M., Kundu S., Rahman M.M., Hannan M.A., Uddin M.J. Prospects of honey in fighting against COVID-19: Pharmacological insights and therapeutic promises. Heliyon. 2020;6:e5798. doi: 10.1016/j.heliyon.2020.e05798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand S., Deighton M., Livanos G., Pang E.C.K., Mantri N. Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci. Rep. UK. 2019;9:18197. doi: 10.1038/s41598-019-54679-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escuredo O., Silva L.R., Valentão P., Seijo M.C., Andrade P.B. Assessing rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012;130:671–678. doi: 10.1016/j.foodchem.2011.07.107. [DOI] [Google Scholar]
- 7.Brudzynski K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006;52:1228–1237. doi: 10.1139/w06-086. [DOI] [PubMed] [Google Scholar]
- 8.Tuksitha L., Chen Y.-L.S., Chen Y., Wong K.-Y., Peng C.-C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak) J. Asia-Pac. Èntomol. 2018;21:563–570. doi: 10.1016/j.aspen.2018.03.007. [DOI] [Google Scholar]
- 9.Brudzynski K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020;332:127229. doi: 10.1016/j.foodchem.2020.127229. [DOI] [PubMed] [Google Scholar]
- 10.Mavric E., Wittmann S., Barth G., Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008;52:483–489. doi: 10.1002/mnfr.200700282. [DOI] [PubMed] [Google Scholar]
- 11.Adams C.J., Boult C.H., Deadman B.J., Farr J.M., Grainger M.N., Manley-Harris M., Snow M.J. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2008;343:651–659. doi: 10.1016/j.carres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Cianciosi D., Forbes-Hernández T.Y., Afrin S., Gasparrini M., Reboredo-Rodriguez P., Manna P.P., Zhang J., Bravo Lamas L., Martínez Flórez S., Agudo Toyos P., et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules. 2018;23:2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isla M.I., Craig A., Ordoñez R., Zampini C., Sayago J., Bedascarrasbure E., Alvarez A., Salomón V., Maldonado L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT-Food Sci. Technol. 2011;44:1922–1930. doi: 10.1016/j.lwt.2011.04.003. [DOI] [Google Scholar]
- 14.Jibril F.I., Hilmi A.B.M., Manivannan L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019;43:4. doi: 10.1186/s42269-019-0044-7. [DOI] [Google Scholar]
- 15.Zhou J., Yao L., Li Y., Chen L., Wu L., Zhao J. Floral classification of honey using liquid chromatography-diode array detection-tandem mass spectrometry and chemometric analysis. Food Chem. 2014;145:941–949. doi: 10.1016/j.foodchem.2013.08.117. [DOI] [PubMed] [Google Scholar]
- 16.Machado A.M., Miguel M.G., Vilas-Boas M., Figueiredo A.C. Honey volatiles as a fingerprint for botanical origin—A review on their occurrence on monofloral honeys. Molecules. 2020;25:374. doi: 10.3390/molecules25020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth G., Lemberkovics E., Kutasi-Szabo J. The volatile components of some Hungarian honeys and their antimicrobial effects. Am. Bee J. 1987;127:496–497. [Google Scholar]
- 18.Acevedo F., Torres P., Oomah B.D., De Alencar S.M., Massarioli A.P., Martín-Venegas R., Albarral-Ávila V., Burgos-Díaz C., Ferrer R., Rubilar M. Volatile and non-volatile/semi-volatile compounds and in vitro bioactive properties of Chilean Ulmo (Eucryphia cordifolia Cav.) honey. Food Res. Int. 2017;94:20–28. doi: 10.1016/j.foodres.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Majtan J., Klaudiny J., Bohova J., Kohutova L., Dzurova M., Sediva M., Bartosova M., Majtan V. Methylglyoxal-induced modifications of significant honeybee proteinous components in Manuka honey: Possible therapeutic implications. Fitoterapia. 2012;83:671–677. doi: 10.1016/j.fitote.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Alzahrani H.A., Alsabehi R., Boukraâ L., Abde-Llah F., Bellik Y., Bakhotmah B.A. Antibacterial and Antioxidant Potency of Floral Honeys from Different Botanical and Geographical Origins. Molecules. 2012;17:10540–10549. doi: 10.3390/molecules170910540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taormina P.J., Niemira B., Beuchat L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001;69:217–225. doi: 10.1016/S0168-1605(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 22.Matzen R.D., Zinck Leth-Espensen J., Jansson T., Nielsen D.S., Lund M.N., Matzen S. The antibacterial effectin vitro of honey derived from various Danish flora. Dermatol. Res. Pract. 2018;2018:1–10. doi: 10.1155/2018/7021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar N.D. Comparison of the antibacterial efficacy of Manuka honey against E. faecalis and E. coli—An in vitro study. J. Clin. Diagn. Res. 2014;8:29–31. doi: 10.7860/JCDR/2014/9676.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulasidas S., Rao P., Bhat S., Manipura R. A study on biofilm production and antifungal drug resistance among Candida species from vulvovaginal and bloodstream infections. Infect. Drug Resist. 2018;11:2443–2448. doi: 10.2147/IDR.S179462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lusby P.E., Coombes A.L., Wilkinson J.M. Bactericidal Activity of Different Honeys against Pathogenic Bacteria. Arch. Med. Res. 2005;36:464–467. doi: 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins R., Roberts A., Brown H.L. On the antibacterial effects of manuka honey: Mechanistic insights. Res. Rep. Biol. 2015;6:215–224. doi: 10.2147/RRB.S75754. [DOI] [Google Scholar]
- 27.Dżugan M., Tomczyk M., Sowa P., Grabek-Lejko D. Antioxidant Activity as Biomarker of Honey Variety. Molecules. 2018;23:2069. doi: 10.3390/molecules23082069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng J., Liu R., Lu Q., Hao P., Xu A., Zhang J., Tan J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to Manuka honey. Food Chem. 2018;252:243–249. doi: 10.1016/j.foodchem.2018.01.115. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Wang B., Fu Y., Shi Y., Chen F., Guan H., Liu L., Zhang C., Zhu P., Liu Y., et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2020;346:128880. doi: 10.1016/j.foodchem.2020.128880. [DOI] [PubMed] [Google Scholar]
- 30.Manyi-Loh C.E., Ndip R.N., Clarke A.M. Volatile Compounds in Honey: A Review on Their Involvement in Aroma, Botanical Origin Determination and Potential Biomedical Activities. Int. J. Mol. Sci. 2011;12:9514–9532. doi: 10.3390/ijms12129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed M.E.A. Factors Affecting the Physicochemical Properties and Chemical Composition of Bee’s Honey. Food Rev. Int. 2020:1–12. doi: 10.1080/87559129.2020.1810701. [DOI] [Google Scholar]
- 32.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro R.D.O.R., Carneiro C.D.S., Mársico E.T., Cunha F.L., Junior C.A.C., Mano S.B. Influence of the time/temperature binomial on the hydroxymethylfurfural content of floral honeys subjected to heat treatment. Ciência Agrotecnologia. 2012;36:204–209. doi: 10.1590/S1413-70542012000200009. [DOI] [Google Scholar]
- 34.Pasias I.N., Kiriakou I.K., Proestos C. HMF and diastase activity in honeys: A fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017;229:425–431. doi: 10.1016/j.foodchem.2017.02.084. [DOI] [PubMed] [Google Scholar]
- 35.Ministry of Health of the People’s Republic of China . Method of the Determination of Plant Pollen in Honey. National Standards of People’s Republic of China; Beijing, China: GB/T 23194-2008. [Google Scholar]
- 36.Wang F., Qian N., Zhang Y., Yang H. Pollen Morphology of Chinese Plants. 2nd ed. Science Press; Beijing, China: 1995. [Google Scholar]
- 37.Oelschlaegel S., Gruner M., Wang P.-N., Boettcher A., Koelling-Speer I., Speer K. Classification and Characterization of Manuka Honeys Based on Phenolic Compounds and Methylglyoxal. J. Agric. Food Chem. 2012;60:7229–7237. doi: 10.1021/jf300888q. [DOI] [PubMed] [Google Scholar]
- 38.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999;299C:152–178. [Google Scholar]
- 39.Scherer R., Godoy H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009;112:654–658. doi: 10.1016/j.foodchem.2008.06.026. [DOI] [Google Scholar]
- 40.Atmani D., Chaher N., Berboucha M., Ayouni K., Lounis H., Boudaoud H., Debbache N., Atmani D. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009;112:303–309. doi: 10.1016/j.foodchem.2008.05.077. [DOI] [Google Scholar]
- 41.Wang X., Yang S., He J., Chen L., Zhang J., Jin Y., Zhou J., Zhang Y. A green triple-locked strategy based on volatile-compound imaging, chemometrics, and markers to discriminate winter honey and sapium honey using headspace gas chromatography-ion mobility spectrometry. Food Res. Int. 2019;119:960–967. doi: 10.1016/j.foodres.2019.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within the article.