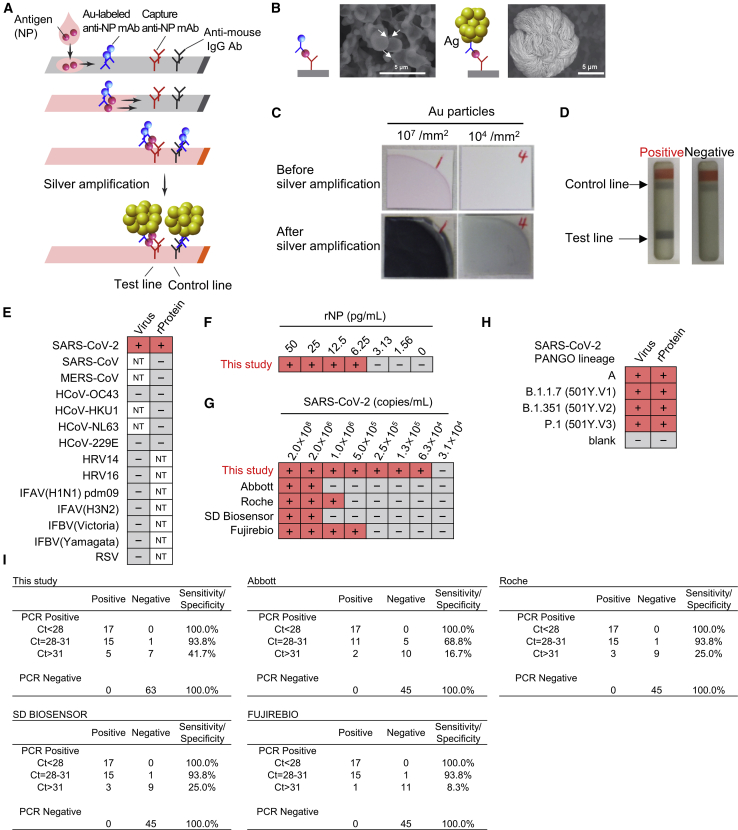
Figure 4.
Immunochromatographic test using developed monoclonal antibodies with silver amplification technology
(A) Schematic diagram of lateral flow immunoassay with silver amplification technology. The antigen in the sample dropped into the device flows on the cellulose membrane together with the colloidal gold-labeled anti-SARS-CoV-2 NP antibody, and when captured by the membrane-immobilized capture antibody, it develops color and appears as a single band. Adherence of silver ions to the surface of a catalytic gold nanoparticle causes electrons to reduce the silver atoms, leading to the size enhancement followed by 1,000-fold improvement in visibility.
(B and C) Size differences in SEM images (B) and naked eye visualized bands (C) with and without silver amplification.
(D) Representative test result for positive and negative.
(E) Specificity of LFIA. Simulated specimens positive for indicated viruses prepared by adding recombinant protein or common respiratory viruses to pooled COVID-19 RT-PCR-negative specimens were analyzed by LFIA. Virus indicates inactivated virus (at least 106 copies/mL for HCoV-229E and HCoV-OC43 or at least 105 TCID50/mL for other viruses). “Protein” indicates recombinant NP antigen of corresponding virus (200 ng/mL). NT, not tested (two technical replicates).
(F and G) Detection limit of LFIA. Recombinant protein (F) and inactivated SARS-CoV-2 (G) were subjected to LFIA analysis. Detection limit using SARS-CoV-2 was compared with indicated antigen detection kits. + and − indicate positive and negative detection, respectively (two technical replicates).
(H) Detection of variant strains of SARS-CoV-2. Virus indicates inactivated virus (less than 1.5 × 106 copies/mL). Protein indicates recombinant NP antigen from each strain (less than 100 pg/mL; two technical replicates).
(I) Sensitivity and specificity of indicated SARS-CoV-2 antigen-detection kits in PCR-positive (n = 45) and negative (n = 63 for this study; n = 45 for the others) specimens in nasopharyngeal swabs.