ABSTRACT
Introduction
Exercise-induced laryngeal obstruction (EILO) is a differential diagnosis for asthma and prevalent in athletes referred for exercise-induced dyspnea. The aim of this study was to estimate the prevalence of EILO in elite cross-country skiers, known for a high prevalence of asthma.
Method
Elite cross-country skiers were invited for screening of EILO. Screening consisted of clinical assessment, questionnaires, skin prick test, spirometry, eucapnic voluntary hyperventilation test, and continuous laryngoscopy during exercise test. Current asthma was defined as physician-diagnosed asthma and use of asthma medication during the last 12 months. EILO was defined as ≥2 points at the supraglottic or glottic level during exercise at maximal effort, using a visual grade score system.
Result
A total of 89 (51% female) cross-country skiers completed the study. EILO was identified in 27% of the skiers, 83% of whom were female. All skiers with EILO had supraglottic EILO, and there was no glottic EILO. Current asthma was present in 34 (38%) of the skiers, 10 (29%) of whom had concomitant EILO. In the skiers with EILO, a higher proportion reported wheeze or shortness of breath after exercise, compared with skiers without EILO. In skiers with EILO and current asthma, compared with skiers with asthma only, a higher proportion reported wheeze or shortness of breath after exercise. Asthma medication usage did not differ between these groups.
Conclusion
EILO is common in elite cross-country skiers, especially females. Asthma and EILO may coexist, and the prevalence of respiratory symptoms is higher in skiers with both. Testing for EILO should be considered in cross-country skiers with respiratory symptoms.
Key Words: SPORT, ATHLETE, EIB, ASTHMA, EILO, CLE
Exercise-induced dyspnea (EID) in the elite athlete has been associated with exercise-induced asthma. Asthma is common in endurance sport athletes (1). In cross-country skiers, 27% report physician-diagnosed asthma (2). However, an important differential diagnosis in athletes with EID is exercise-induced laryngeal obstruction (EILO), involving a transient laryngeal obstruction at either glottic (i.e., vocal cords) or supraglottic level during high-intensity exercise (3). This airflow obstruction may result in symptoms such as wheeze, shortness of breath, and stridor (4,5). A standardized test for diagnosing EILO used in a clinical and research setting is the continuous laryngoscopy during exercise (CLE) test. This test uses a flexible nasolaryngoscope to continuously visualize the larynx throughout a maximal effort exercise bout, to enable identification and characterization of a laryngeal obstruction (6,7).
To the best of our knowledge, the prevalence of EILO has never been investigated within a population of athletes who were not preselected for respiratory symptoms. The prevalence of EILO in the general population has been estimated at 5.7%–7.6% (8,9). In athletes with respiratory symptoms referred to a tertiary asthma clinic for examination, EILO was found in 35%–70%, sometimes with asthma and sometimes without evidence of asthma despite asthma diagnosis and treatment (3,10). Given that symptoms of EILO mimic asthma and that asthma treatment has no effect on EILO, undiagnosed EILO in an athlete using asthma treatment may result in unnecessary or ineffective interventions.
Cross-country skiers and biathletes have a very high prevalence of asthma and exercise-induced respiratory symptoms (2,11). The increased prevalence of asthma in cross-country skiers is mainly believed to be due to repeated and prolonged inhalation of cold dry air, leading to osmotic changes and epithelial damage in the airways (12). However, one study found that only 21% of Swedish elite cross-country skiers under treatment for asthma had evidence of bronchial hyperreactivity (BHR) (13), indicating a possible overdiagnosis of asthma in this particular population.
More information is needed about the prevalence of EILO in competitive athletes given that it is prevalent in those with respiratory symptoms and may be mistaken for asthma. Therefore, we assessed elite skiers, known for a high prevalence of physician-diagnosed asthma and respiratory symptoms with the aim of estimating the prevalence of EILO.
METHODS
Study design and study population
This screening study was approved by the Regional Ethical Review Board, Umeå, Sweden (Dnr 2015-43-31M). Elite cross-country skiers (competing in cross-country skiing, biathlon, or ski-orienteering) 15–35 yr of age were eligible for inclusion. The skiers belonged to (a) Swedish national teams, (b) national elite upper secondary sport schools, or (c) universities with elite sports agreement or (d) had competed in a Swedish, Nordic, or international championship or an International Biathlon Union/International Skiing Federation competition during the last 12 months. Information about the study and recruitment was distributed to coaches and the national teams’ medical staff, and also via poster advertisement at suitable facilities.
Study procedures
The study was carried out at the Clinical Research Centre and ENT department at Östersund Hospital, Sweden, 2015 to 2017. After receiving information and giving written consent, participants underwent a thorough medical history, clinical examination, dynamic spirometry, eucapnic voluntary hyperventilation (EVH) test, skin prick test, and CLE test and completed questionnaires. Length and weight were recorded, and body mass index was calculated (kg·m−2).
Dynamic spirometry was conducted using Spirare 3 (Diagnostics, Norway) or Jaeger Vyntus IOS (CareFusion, Germany) according to ERS/ATS guidelines (14), using predictive equations for spirometry by Hedenström et al.(15,16). EVH testing was done using the AILOS Asthma Test (Ailos Medical AB, Karlstad, Sweden) or Eucapsys (SMTEC, Switzerland). In short, participants hyperventilated dry air containing 5% CO2 (Carboair, Air Liquid GAS, Sweden) for 6 min (17). Target minute ventilation was set to 85% of maximum voluntary ventilation (MVV) (30 × forced expiratory volume in 1 s [FEV1]). The EVH test was considered invalid if average minute ventilation was <60% of MVV (21 × FEV1) (18). FEV1 was measured before and at 3, 5, 10, 15, and 20 min after EVH, with the highest FEV1 of two approved maneuvers recorded. BHR was defined as a ≥10% decrease in FEV1 after EVH (19). Participants were instructed to avoid vigorous exercise, heavy meals, caffeine, or nicotine for 8 h before the EVH test. They also were instructed to refrain from using asthma medication before the test, within the following time frames: short-acting β2 agonists (SABA) for 8 h, inhaled corticosteroids (ICS) for 12 h, long-acting β2 agonists (LABA) for 24 h, and leukotriene receptor agonists (LTRA) for 3 d. Participants had to be free from respiratory infection for at least 6 wk before the test.
An intracutaneous skin prick test was performed according to European standards with extracts of airborne allergens using ALK-Abello Soluprick (ALK, Sweden) (20). A ≥3-mm weal after 15 min was considered a positive result. Allergy was defined as self-reported allergic symptoms and a positive skin prick test.
Participants were asked to complete a questionnaire based on the ECRHS II, with additional questions regarding exercise (h·wk−1) and sport (21), and the GerdQ questionnaire (22). Current asthma was defined as self-reported physician-diagnosed asthma and the use of asthma medication during the last 12 months. Respiratory symptoms were defined as a positive answer to having either wheeze without having a cold or shortness of breath after exercise, during the last 12 months. Gastroesophageal reflux disease (23) was defined as 8 points or more on the GerdQ questionnaire (22). A healthcare contact was defined as having sought medical consultation because of respiratory difficulties or breathlessness during the last 12 months. The use of asthma medication was presented as daily use of bronchodilators (SABA or LABA) or daily use of combination therapy (SABA/LABA and ICS or LTRA).
Continuous laryngoscopy during exercise test
CLE was performed according to Heimdal et al. (6) and Maat et al. (7). The larynx was visualized with nasal fiber-optic laryngoscopy (FNL-7RP3; Pentax, Tokyo, Japan) and video recorded during exercise on a treadmill (Intelligent Suspension 3; Cybex, Rosemont, IL). Naphazoline–lidocaine was used for local anesthesia and dilatation of the nasal cavity. Speed and/or inclination of the treadmill was increased at every minute after a 3-min warm-up to reach maximal effort after 6–9 min of exercise. Speed increased by 1 km·h−1 up to a maximum of 15 km·h−1 and inclination increased by 2% up to a maximum of 8%. Subjects wore a safety harness system attached to the ceiling. Heart rate was monitored using a chest strap (Polar Electro, Kempele, Finland). The CLE test was considered valid if the participant reached >90% of estimated heart rate (24).
Laryngeal obstruction at the glottic and supraglottic levels was assessed at 1 min after warm-up and at maximal effort, using a visual grade score (0–3 points) according to Maat et al. (7). EILO was defined as ≥2 points at the supraglottic or glottic level at maximal effort, in accordance with other studies on EILO (8,9,25). A senior ENT physician experienced in CLE testing (M.R.) performed the CLE test, accompanied by a physiotherapist (C.B.). This physician graded the laryngeal obstruction, blinded to results from other parts of the study and to whether the participant had airway symptoms. A second blinded grading of the video laryngoscopies was done by L.N. or T.I. for calculation of interrater agreement. A final score was set by M.R. in case of disagreement.
Using ordinal numbers, the observers (C.B. and M.R.) graded respiratory distress during CLE as “none”; “mild, with audible respiration”; “moderate, with stridor”; and “collapse or panic attack.” Any disagreement in grading was solved by reaching consensus. Immediately after stopping the exercise at the CLE test, participants were asked to rate their perceived physical exertion according to the Borg scale, ranging from 6 (no exertion at all) to 20 (maximal exertion) (26).
In total, 7 (7%) of the 98 skiers discontinued the CLE test before exercise or during warm-up because of discomfort (n = 3) or vasovagal reaction (n = 4), one of whom fainted (Fig. 1). There were no injuries in association with the CLE test. One skier terminated the CLE test because of exhaustion at peak heart rate 172 bpm (89.1% of estimated maximum heart rate). The skier did not have respiratory symptoms as graded by observer, scored 17 on the Borg scale, and was not excluded.
FIGURE 1.
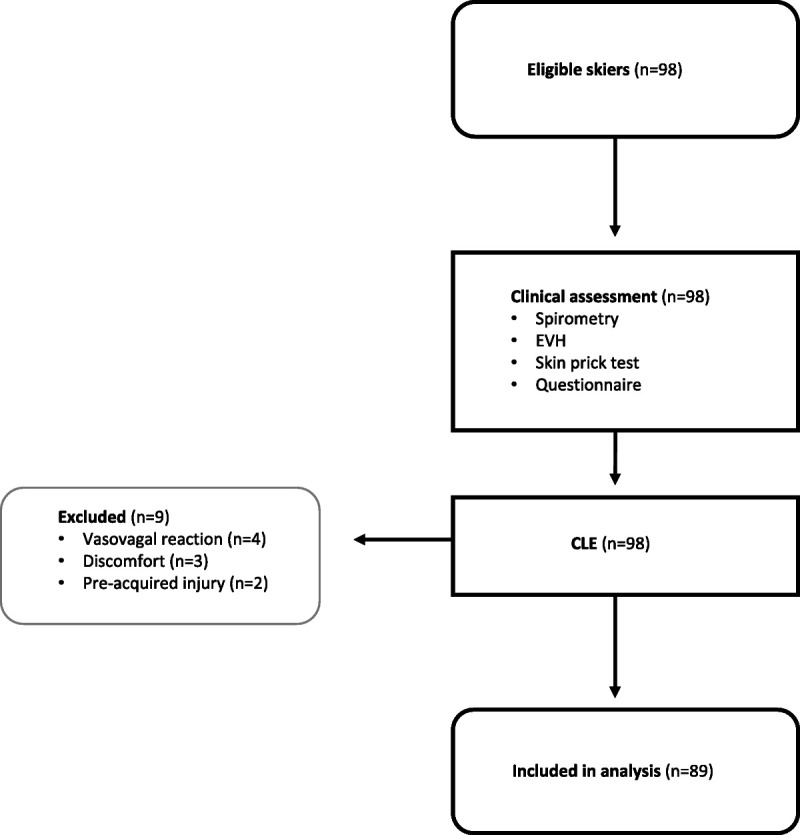
Flowchart of inclusion and exclusion in study.
Statistical analyses
All analyses were conducted using R Statistical Software (version 3.6.1). The primary outcome was prevalence of EILO. A sample size of 100 participating athletes and an a priori estimated prevalence of EILO at 15% (95% confidence interval, 9%–24%) were calculated. The chi-square test and t-test were used to analyze categorical and continuous variables as appropriate. Fisher’s exact test was used to analyze categorical variables if the number of observations was too small. Interrater agreement was calculated using Cohen’s kappa with linear weights (27). A P value of 0.05 or less was considered significant.
RESULTS
Description of participants
In total, 98 skiers were assessed, and 89 completed all study procedures (Fig. 1). The study population had an even sex distribution, and all participants were under 31 yr of age (Table 1). The age distribution was similar between the sexes. Current asthma and exercise-induced respiratory symptoms were common. No skier had a previous diagnosis of EILO according to medical history. Of the 89 skiers, 82 (92%) reached a threshold value of >60% MVV at the EVH test. Five participants hyperventilated <60% of MVV, and two test results were missing because of equipment failure. In total, 24 skiers reached target ventilation 85% of MVV. BHR was present in 6 (7.3%) of the skiers with valid EVH. Of these six, two had current asthma and four had no previous asthma diagnosis or medication. Furthermore, four of the skiers with BHR reported no exercise-related respiratory symptoms.
TABLE 1.
A description of 89 Swedish elite cross-country skiers screened for EILO.
| Males (n = 44) | Females (n = 45) | Total (N = 89) | |
|---|---|---|---|
| Age, median (min–max) | 18 (16 to 27) | 18 (15 to 30) | 18 (15 to 30) |
| Training (h·wk−1) | 12 (10 to 14) | 12 (10 to 14) | 12 (10 to 14) |
| Body mass index (kg·m−2) | 22.6 (21.5 to 23.3) | 21.5 (20.4 to 23) | 22.1 (20.8 to 23.1) |
| FEV1 (L) | 4.71 (4.42 to 5.10) | 3.72 (3.45 to 3.97) | 4.16 (3.67 to 4.74) |
| FEV1 (% of predicted) | 106.1 (101 to 112.8) | 101.2 (95.0 to 112.0) | 103.0 (97.0 to 112.8) |
| FVC (L) | 5.7 (5.2 to 6.1) | 4.2 (3.9 to 4.7) | 4.9 (4.2 to 5.7) |
| FVC (% of predicted) | 101.0 (96.0 to 111.0) | 98.5 (91.9 to 107.2) | 100.6 (95.0 to 109.0) |
| FEV1/FVC | 0.84 (0.81 to 0.88) | 0.87 (0.84 to 0.90) | 0.86 (0.82 to 0.89) |
| FEV1 after EVH (%)a | −4.5 (−7.3% to −3.3%) | −3.8% (−6.2% to −2.2%) | −4.2% (−6.7% to −2.7%) |
| Ventilated volume during EVH (L) | 762 (702 to 883) | 607 (570 to 666) | 684 (602 to 761) |
| Ventilated volume during EVH (% of MVV) | 80.6 (72.0 to 86.1) | 77.5 (71.3 to 85.5) | 79.6 (71.4 to 85.9) |
| Respiratory symptoms,b n (%) | 14 (32) | 23 (51) | 37 (42) |
| Wheeze without having a cold, n (%) | 11 (25) | 18 (40) | 29 (33) |
| Shortness of breath after exercise, n (%) | 10 (23) | 14 (31) | 24 (27) |
| Current asthma,c n (%) | 16 (36) | 18 (40) | 34 (38) |
| Allergy,d n (%) | 16 (38) | 13 (30) | 29 (34) |
| GERD,e n (%) | 6 (14) | 0 (0) | 6 (7) |
Data presented as median (IQR) if not stated otherwise.
aMaximal % change compared with baseline.
bRespiratory symptoms defined as the presence of either wheeze without having a cold or shortness of breath after exercise.
cCurrent asthma defined as physician-diagnosed asthma and use of asthma medication in the last 12 months.
dAllergy defined as positive skin prick test and symptomatic allergic rhinitis.
eGastroesophageal reflux disease defined as 8 points or more on GerdQ questionnaire (21).
FVC, forced vital capacity.
EILO
EILO, defined as a supraglottic or glottic visual grade score of ≥2, was present in 24 (27%) of the 89 skiers, all of whom had supraglottic EILO; no skier had glottic EILO. A detailed presentation of the visual grade scores is shown in Table 2. A comparison between skiers with and without EILO is shown in Table 3. In the 24 skiers with EILO, 20 were females, and 19 (83%) had either self-reported respiratory symptoms during the last 12 months or respiratory distress during CLE, as graded by observer. Observer-graded respiratory distress during CLE test was significantly more common in skiers with EILO that in skiers without EILO (48% vs 6%, P < 0.001); all but one had “mild, with audible respiration,” and one skier with EILO has “moderate, with stridor.” In the skiers with GERD, one out of six had EILO. There was no significant association between GERD and EILO (odds ratio, 0.57; 95% confidence interval, 0.01–5.49). Median (interquartile range) heart rate during the CLE test was 95.3% (92.3%–98.4%) of peak heart rate. There was no difference in exertion according to Borg scale scores between skiers with and without EILO (median [range], 16.5 [15–17.5] vs 17 [12–19]; P = 0.856). In total, four skiers rated their exertion below 15 (min–max, 12–14). All four had a valid CLE test, did not have observer-graded respiratory distress, and did not fulfill the study criterion for EILO. Interrater agreement of supraglottic scores ranged from fair to moderate (Cohen’s κ, 0.34 [M.R. and L.N.] to 0.46 [M.R. and T.I.]).
TABLE 2.
Supraglottic and glottic CLE scores at maximal effort in 89 elite skiers.
| Supraglottic Score | Total | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Glottic score | 0 | 19 | 45 | 23 | 87 | |
| 1 | 1 | 1 | 2 | |||
| 2 | 0 | |||||
| 3 | 0 | |||||
| Total | 19 | 46 | 24 | 0 | ||
Gray areas distinguish CLE scores resulting in EILO, defined as CLE score of ≥2 at supraglottic or glottic level at maximal effort.
TABLE 3.
A description and comparison of 89 Swedish elite skiers screened for EILO.
| EILO (n = 24) | No EILO (n = 65) | P | |
|---|---|---|---|
| Females | 20 (83) | 25 (38) | <0.001 |
| Age, median (IQR) | 18 (17–20.25) | 18 (17–19) | 0.537 |
| Respiratory distress during CLEa | 11 (48) | 4 (6) | <0.001 |
| Respiratory symptomsb | 15 (62) | 22 (34) | 0.015 |
| Wheeze without having a cold | 12 (50) | 17 (26) | 0.033 |
| Shortness of breath after exercise | 12 (50) | 12 (18) | 0.003 |
| Current asthmac | 10 (42) | 24 (37) | 0.683 |
Data presented as n (%) if not stated otherwise. Significant P values are presented in bold.
aObserver-graded respiratory distress during CLE test.
bRespiratory symptoms defined as the presence of either wheeze without having a cold or shortness of breath after exercise.
cPhysician-diagnosed asthma and use of asthma medication in the last 12 months.
Asthma and EILO
Current asthma was present in 34 skiers (38%). Concurrent asthma and EILO were common in the skiers (Fig. 2). A comparison of skiers with asthma versus asthma and EILO is presented in Table 4. In skiers with current asthma, 10 (29%) had EILO. In skiers with current asthma and concomitant EILO, a higher proportion reported exercise-related respiratory symptoms compared with those without concomitant EILO (100% vs 62%, P = 0.024). The proportion of skiers using daily bronchodilator medication was not higher in those with current asthma and concomitant EILO compared with skiers with only asthma (44% vs 58%, P = 0.475).
FIGURE 2.
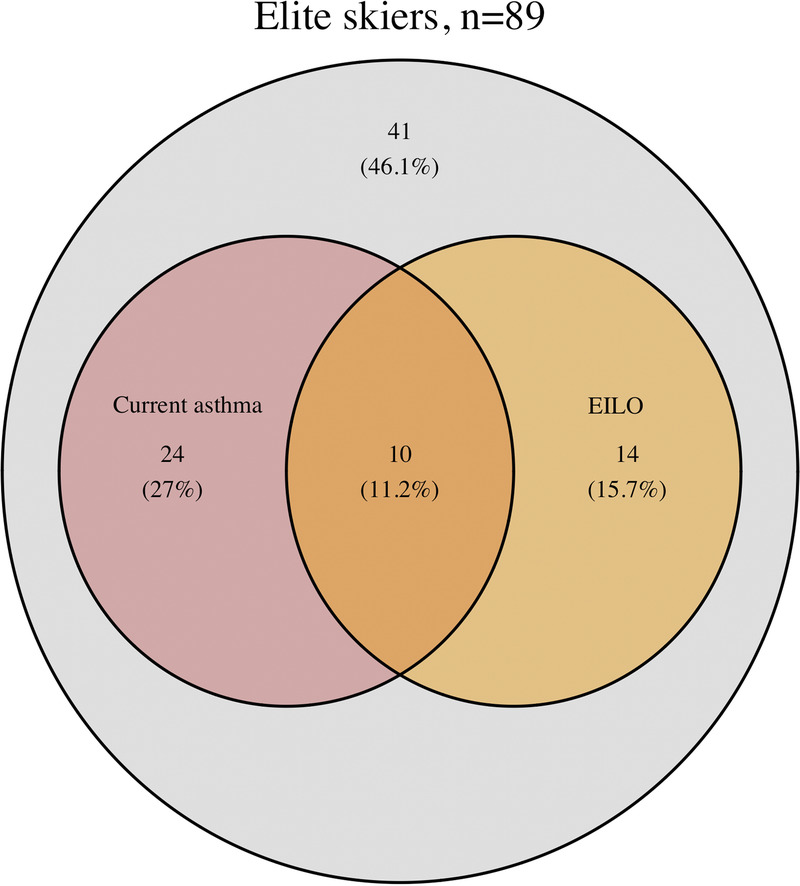
Prevalence of current asthma, defined as physician-diagnosed asthma and use of asthma medication in the last 12 months, and exercise-induced laryngeal obstruction (EILO) diagnosed using the CLE test, within a population of Swedish elite cross-country skiers.
TABLE 4.
A comparison of 34 skiers with current asthma, with and without EILO.
| Current Asthma and EILO (n = 10) | Current Asthma (n = 24) | P | |
|---|---|---|---|
| Female | 8 (80) | 10 (42) | 0.041 |
| Age, median (IQR) | 20 (19–20.75) | 19 (17.75–20.25) | 0.375 |
| Respiratory symptomsa | 10 (100) | 15 (62) | 0.024 |
| Shortness of breath after exercise | 9 (90) | 10 (42) | <0.001 |
| Wheeze without having a cold | 9 (90) | 12 (50) | 0.029 |
| Daily SABA/LABAb | 4 (44) | 14 (58) | 0.475 |
| Daily combination treatmentc | 5 (56) | 15 (62) | 0.716 |
| Healthcare contact last 12 monthsd | 3 (33) | 11 (46) | 0.518 |
Data presented as n (%) if not stated otherwise. Significant P values are presented in bold.
aRespiratory symptoms defined as the presence of either wheeze without having a cold or shortness of breath after exercise.
bDaily use of SABA/LABA during the last 12 months.
cDaily use of SABA/LABA in combination with ICS or LTRA during the last 12 months.
dHealthcare contact because of breathlessness or breathing difficulties during the last 12 months.
DISCUSSION
Screening of EILO in Swedish elite cross-country skiers and biathletes detected 27% with EILO. Skiers with EILO were predominantly female and reported more respiratory symptoms compared with skiers without EILO. It was common to have both asthma and EILO.
EILO
The prevalence of EILO found in Swedish competitive skiers in the current study is considerably higher than the 5.7% to 7.6% prevalence detected within general populations (8,9). However, EILO appears to be even more common, up to 35%–70%, in athletes referred to hospitals for exercise-induced respiratory symptoms (3,8–10).
In the present study, a majority of the skiers with EILO had respiratory symptoms in conjunction with exercise, i.e., “symptomatic EILO.” However, 17% of the skiers that fulfilled study criteria for EILO (≥2 points at the visual grading score) did not report any respiratory symptoms in the last 12 months or had any observer-graded respiratory distress during the CLE test. This is not the first study detecting “asymptomatic EILO.” EILO was diagnosed in 4.8% of asymptomatic individuals in a general adolescent population (4), and in a study from Denmark, 26.2% of those diagnosed with EILO in a general population were asymptomatic (8,9). Self-reported symptoms have been shown to be a poor predictor of EILO (9,25). Furthermore, although obstruction at the laryngeal level predominantly induces inspiratory distress, as opposed to the classic expiratory symptoms of bronchoconstriction, self-reported inspiratory symptoms are of limited value at discerning EILO from exercise-induced bronchoconstriction in athletes (3). By contrast, self-reported symptoms in athletes have low sensitivity and specificity for EIB as well (28,29). Thus, self-reported respiratory symptoms seem to be of limited value at predicting (mild) exercise-related airway obstruction, especially in athletes. Albeit a small pilot study, Walsted et al. found that EILO does lead to increased work of breathing and increased respiratory neural drive although the subjects with EILO tended to report less dyspnea during high-intensity exercise compared with controls (30). Whether mild laryngeal obstruction in an asymptomatic athlete constitutes a disease (i.e., EILO) or a normal laryngeal response to high-intensity exercise needs to be established.
EILO was more common in female skiers. In the skiers with EILO, 83% were female, compared with 38% in those without EILO. This pattern is in line with earlier studies on athletes with respiratory symptoms referred to hospitals, which also showed a higher prevalence of EILO in females (3,10). By contrast, data on EILO distribution between sexes in the general population are conflicting (8,9). An increased risk of EILO has been attributed to a smaller larynx because of young age or female sex (8,9,31). Accordingly, a skewed age distribution between females and males might affect EILO prevalence by sex. However, in the present study, the age distribution between the sexes was even. The preponderance of EILO in females and to what extent larynx size affects risk for EILO require further investigation.
In the present study, all skiers with EILO presented with supraglottic EILO, and no one demonstrated EILO on a glottic level. This result is in keeping with previous studies using the CLE test, in which supraglottic EILO has been the dominant subtype (3,8,31,32). However, studies using methods other than CLE to investigate laryngeal obstruction often identify a higher prevalence of glottic obstruction (10,33). Perhaps glottic EILO is a larger problem for elite cross-country skiers, preventing them from pursuing a career as an elite athlete at an early age, thus explaining the absence of any glottic obstruction in our population. Glottic versus supraglottic EILO is important from a treatment perspective because supraglottic EILO can, in some well-defined severe cases, be treated surgically with supraglottoplasty (34).
Asthma
The current results indicate that asthma may be overdiagnosed in cross-country skiers. Similar to larger studies on Swedish skiers (2,11), the present study population had a high prevalence of self-reported current asthma. However, only 7% of the 82 skiers with valid EVH test had BHR. EVH is widely used to test for BHR and has been recommended by the International Olympic Committee for diagnosing exercise-induced bronchoconstriction in athletes (19). The bronchoconstriction after EVH has a dose–response relationship, and adequate minute ventilation is thus important (17). Indeed, the criterion used to evaluate the respiratory response to EVH affects the results and subsequent diagnosis of BHR (35). The criterion for the diagnosis of BHR used in the present study was a single measurement with a decrease in FEV1 ≥ 10% after EVH as indicative of BHR, as presented by Anderson et al. and recommended by ATS guidelines on EIB (19,36). If the present study would have used a calculation of fall index after EVH that takes minute ventilation into account, it would likely have rendered a higher prevalence of BHR (35). However, there is an ongoing discussion about which criterion to use, with some alternatives being more conservative than the one being used in the present study (35,37). Also, more than one EVH test may be needed to identify mild BHR because of poor diagnostic reproducibility in athletes (38). Another possible explanation for the low prevalence of BHR is that most of those with current asthma used ICS, which can attenuate bronchoconstriction on indirect tests such as EVH (39). In addition, in the present study, four out of six skiers with BHR did not have a previous diagnosis of asthma, and four were asymptomatic, underlining that respiratory symptoms in cross-country skiers are poorly associated with BHR (13).
Asthma and EILO
Coexisting EILO and asthma seem to be common in skiers. Similar findings have been reported within a general population and in populations of athletes with respiratory symptoms referred to hospitals (3,9,10). We found that skiers with both conditions displayed an increased prevalence of exercise-induced respiratory symptoms, but we could not detect differences in the use of daily asthma medication between the groups. Whether athletes with asthma and EILO distinguish themselves in other respects regarding asthma medication was not elucidated in our study and needs to be further investigated.
Testing for both EILO and asthma in skiers with EID should be considered because of the high prevalence of EILO and the prevalent overlap of the conditions. The CLE test is considered a well-tolerated and safe test (6,32). However, the nasal fiberscope can cause discomfort and also fainting in the participant (40,41). In the present study 7 (7%) of the skiers discontinued the CLE test because of vasovagal reaction or discomfort. Most studies, however, lack details describing any discontinuation of the CLE test. To the best of our knowledge, no participant has been injured when performing the test. The potential benefit of a CLE test in athletes with EID seem to outweigh the potential risks, considering the prevalent overlap of asthma and EILO and the importance of correct diagnosis to ensure correct treatment and avoid overtreatment of asthma.
Study limitations
The CLE test used in the present study may have led to underdiagnosing of, especially glottic, EILO. Glottic EILO more often presents at the end of CLE tests (7). In our study, the athletes’ physical effort during CLE testing may not have been sufficient to induce EILO. The athletes were instructed to carry on until reaching >90% of their estimated maximum heart rate. This cutoff level may have been too low considering that a large proportion of the study participants were competing at an international level and used to high-intensity exercise and pushing themselves to their limit. Indeed, a CLE running on a treadmill indoors at room temperature does not fully simulate a cross-country skiing competition outdoors during winter. We chose treadmill running as this the setup in our laboratory. Cross-country skiers are usually very familiar with treadmill tests, as they regularly perform submaximal and maximal tests running or skiing on a treadmill. In the future, we see no major obstacles to perform a CLE on a larger treadmill using roller skis, or perhaps on skis in an outdoor skiing setting, using portable CLE equipment, which has recently been shown to be feasible (42). Furthermore, investigators tend to underestimate glottic angle during CLE tests (31). Hence, exercise testing with increased effort, with comparison to their intensity during competition, would perhaps show increasing laryngeal obstruction, especially at the glottic level.
By contrast, the high prevalence of EILO can be a consequence of participation bias. The study population was recruited via open invitation and may have attracted predominantly athletes with EID, leading to a risk of overestimating the prevalence of EILO and asthma. Information about the study was mainly distributed via coaches and medical staff, who may have urged especially those with respiratory symptoms or athletes with therapy-resistant asthma to participate in the study. Indeed, cross-country skiers in our study population displayed a high prevalence of respiratory symptoms and current asthma, indicating a possibility of participation bias.
A diagnostic test should be reliable. CLE is a standardized test to investigate EILO using a validated visual grade score system to evaluate the laryngeal obstruction (7,43). This setup has been used in thousands of patients in multiple centers for over a decade (41,43). However, with respect to the visual grading score, a discrepancy between raters may have implications for identifying the presence and severity of EILO. In the present study, the interrater variability of the CLE test was only fair to moderate (44). Previous studies have reported a variable interrater agreement of the visual grade score, ranging from a slight to perfect level of agreement between raters (3,7,45). In our study, video recordings were not preselected based on video quality. Furthermore, the blinded raters had access only to laryngeal video recordings and not to sound or external video recordings of the CLE tests, possibly contributing to the low interrater agreement. Still, the variable interrater agreement indicates the need for a more objective grading system to assess laryngeal obstruction. Addressing this issue, a recent study found that an objective computerized system can quantify laryngeal movements and thus aid EILO diagnosing (46). This is a promising next step and, when validated and clinically available, may provide a significant improvement to the CLE test.
CONCLUSIONS
The present findings suggest that EILO is common in elite cross-country skiers, especially females, and often coexists with asthma. Skiers with EILO and concurrent asthma report more exercise-induced respiratory symptoms than skiers without EILO and/or asthma. This study supports the notion that testing for both EILO and asthma is warranted in athletes with EID.
Acknowledgments
The authors thank the athletes for their participation in the study and also their coaches for their cooperation and help with facilitating athletes’ participation. The authors also thank Anna Lindam for statistical support. This study was financially supported by the School of Sport Sciences, Umeå University; the Visare Norr Fund, Northern County Councils’ Federation; and the Research and Development Unit, Region Jämtland Härjedalen.
The authors have no conflicts of interests to declare relevant to this manuscript. The results in the present study do not constitute endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Contributor Information
CATHARINA BÄCKLUND, Email: catharina.backlund@regionjh.se.
LEIF NORDANG, Email: leifnordang@hotmail.com.
MARIE RYDING, Email: marie.ryding@gmail.com.
NIKOLAI STENFORS, Email: nikolai.stenfors@umu.se.
REFERENCES
- 1.Carlsen KH Anderson SD Bjermer L, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the joint task force of the European Respiratory Society (ERS) and the European academy of allergy and clinical immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63(4):387–403. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson LM, Irewall T, Lindberg A, Stenfors N. Prevalence, age at onset, and risk factors of self-reported asthma among Swedish adolescent elite cross-country skiers. Scand J Med Sci Sports. 2018;28(1):180–6. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc. 2013;45(11):2030–5. [DOI] [PubMed] [Google Scholar]
- 4.Hall A, Thomas M, Sandhu G, Hull JH. Exercise-induced laryngeal obstruction: a common and overlooked cause of exertional breathlessness. Br J Gen Pract. 2016;66(650):e683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shay EO, Sayad E, Milstein CF. Exercise-induced laryngeal obstruction (EILO) in children and young adults: from referral to diagnosis. Laryngoscope. 2020;130(6):E400–6. [DOI] [PubMed] [Google Scholar]
- 6.Heimdal JH, Roksund OD, Halvorsen T, Skadberg BT, Olofsson J. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope. 2006;116(1):52–7. [DOI] [PubMed] [Google Scholar]
- 7.Maat RC Roksund OD Halvorsen T, et al. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol. 2009;266(12):1929–36. [DOI] [PubMed] [Google Scholar]
- 8.Christensen PM, Thomsen SF, Rasmussen N, Backer V. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol. 2011;268(9):1313–9. [DOI] [PubMed] [Google Scholar]
- 9.Johansson H Norlander K Berglund L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax. 2015;70(1):57–63. [DOI] [PubMed] [Google Scholar]
- 10.Hanks CD Parsons J Benninger C, et al. Etiology of dyspnea in elite and recreational athletes. Phys Sportsmed. 2012;40(2):28–33. [DOI] [PubMed] [Google Scholar]
- 11.Norqvist J, Eriksson L, Soderstrom L, Lindberg A, Stenfors N. Self-reported physician-diagnosed asthma among Swedish adolescent, adult and former elite endurance athletes. J Asthma. 2015;52(10):1046–53. [DOI] [PubMed] [Google Scholar]
- 12.Kippelen P, Anderson SD. Pathogenesis of exercise-induced bronchoconstriction. Immunol Allergy Clin North Am. 2013;33(3):299–312, vii. [DOI] [PubMed] [Google Scholar]
- 13.Stenfors N. Self-reported symptoms and bronchial hyperresponsiveness in elite cross-country skiers. Respir Med. 2010;104(11):1760–3. [DOI] [PubMed] [Google Scholar]
- 14.Miller MR Hankinson J Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 15.Hedenström H, Malmberg P, Agarwal K. Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21(6):551–7. [PubMed] [Google Scholar]
- 16.Hedenström H, Malmberg P, Fridriksson HV. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 1986;91(3):299–310. [DOI] [PubMed] [Google Scholar]
- 17.Argyros GJ, Roach JM, Hurwitz KM, Eliasson AH, Phillips YY. Eucapnic voluntary hyperventilation as a bronchoprovocation technique: development of a standarized dosing schedule in asthmatics. Chest. 1996;109(6):1520–4. [DOI] [PubMed] [Google Scholar]
- 18.Brummel NE, Mastronarde JG, Rittinger D, Philips G, Parsons JP. The clinical utility of eucapnic voluntary hyperventilation testing for the diagnosis of exercise-induced bronchospasm. J Asthma. 2009;46(7):683–6. [DOI] [PubMed] [Google Scholar]
- 19.Anderson SD, Argyros GJ, Magnussen H, Holzer K. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br J Sports Med. 2001;35(5):344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzerling L Mari A Bergmann KC, et al. The skin prick test—European standards. Clin Transl Allergy. 2013;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Community Respiratory Health Survey II Steering Committee . The European Community respiratory health survey II. Eur Respir J. 2002;20(5):1071–9. [DOI] [PubMed] [Google Scholar]
- 22.Jones R Junghard O Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30(10):1030–8. [DOI] [PubMed] [Google Scholar]
- 23.Bai Y Du Y Zou D, et al. Gastroesophageal reflux disease questionnaire (GerdQ) in real-world practice: a national multicenter survey on 8065 patients. J Gastroenterol Hepatol. 2013;28(4):626–31. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6. [DOI] [PubMed] [Google Scholar]
- 25.Walsted ES, Hull JH, Sverrild A, Porsbjerg C, Backer V. Bronchial provocation testing does not detect exercise-induced laryngeal obstruction. J Asthma. 2017;54(1):77–83. [DOI] [PubMed] [Google Scholar]
- 26.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 27.Cohen J. Weighted Kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–20. [DOI] [PubMed] [Google Scholar]
- 28.Langdeau JB, Day A, Turcotte H, Boulet LP. Gender differences in the prevalence of airway hyperresponsiveness and asthma in athletes. Respir Med. 2009;103(3):401–6. [DOI] [PubMed] [Google Scholar]
- 29.Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33(2):208–13. [DOI] [PubMed] [Google Scholar]
- 30.Walsted ES Faisal A Jolley CJ, et al. Increased respiratory neural drive and work of breathing in exercise-induced laryngeal obstruction. J Appl Physiol (1985). 2018;124(2):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norlander K, Johansson H, Emtner M, Janson C, Nordvall L, Nordang L. Differences in laryngeal movements during exercise in healthy and dyspnoeic adolescents. Int J Pediatr Otorhinolaryngol. 2020;129:109765. [DOI] [PubMed] [Google Scholar]
- 32.Roksund OD, Maat RC, Heimdal JH, Olofsson J, Skadberg BT, Halvorsen T. Exercise induced dyspnea in the young. Larynx as the bottleneck of the airways. Respir Med. 2009;103(12):1911–8. [DOI] [PubMed] [Google Scholar]
- 33.Tilles SA, Ayars AG, Picciano JF, Altman K. Exercise-induced vocal cord dysfunction and exercise-induced laryngomalacia in children and adolescents: the same clinical syndrome? Ann Allergy Asthma Immunol. 2013;111(5):342–346.e1. [DOI] [PubMed] [Google Scholar]
- 34.Siewers K, Backer V, Walsted ES. A systematic review of surgical treatment for supraglottic exercise-induced laryngeal obstruction. Laryngoscope Investig Otolaryngol. 2019;4(2):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch S, Sinden SM, Koehle MS. Inconsistent calculation methodology for the eucapnic voluntary hyperpnoea test affects the diagnosis of exercise-induced bronchoconstriction. BMJ Open Respir Res. 2018;5(1):e000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons JP Hallstrand TS Mastronarde JG, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187(9):1016–27. [DOI] [PubMed] [Google Scholar]
- 37.Price OJ Ansley L Levai IK, et al. Eucapnic voluntary hyperpnea testing in asymptomatic athletes. Am J Respir Crit Care Med. 2016;193(10):1178–80. [DOI] [PubMed] [Google Scholar]
- 38.Price OJ, Ansley L, Hull JH. Diagnosing exercise-induced bronchoconstriction with eucapnic voluntary hyperpnea: is one test enough? J Allergy Clin Immunol Pract. 2015;3(2):243–9. [DOI] [PubMed] [Google Scholar]
- 39.Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Effect of inhaled budesonide on bronchial reactivity to histamine, exercise, and eucapnic dry air hyperventilation in patients with asthma. Thorax. 1991;46(11):811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson H Norlander K Alving K, et al. Exercise test using dry air in random adolescents: temporal profile and predictors of bronchoconstriction. Respirology. 2016;21(2):289–96. [DOI] [PubMed] [Google Scholar]
- 41.Halvorsen T Walsted ES Bucca C, et al. Inducible laryngeal obstruction: an official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J. 2017;50(3):1602221. [DOI] [PubMed] [Google Scholar]
- 42.Hull JH, Walsted ES, Orton CM, Williams P, Ward S, Pavitt MJ. Feasibility of portable continuous laryngoscopy during exercise testing. ERJ Open Res. 2019;5(1):00219–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roksund OD, Heimdal JH, Clemm H, Vollsaeter M, Halvorsen T. Exercise inducible laryngeal obstruction: diagnostics and management. Paediatr Respir Rev. 2017;21:86–94. [DOI] [PubMed] [Google Scholar]
- 44.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 45.Walsted ES, Hull JH, Hvedstrup J, Maat RC, Backer V. Validity and reliability of grade scoring in the diagnosis of exercise-induced laryngeal obstruction. ERJ Open Res. 2017;3(3):00070–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J, Walsted ES, Backer V, Hull JH, Elson DS. Quantification and analysis of laryngeal closure from endoscopic videos. IEEE Trans Biomed Eng. 2019;66(4):1127–36. [DOI] [PubMed] [Google Scholar]


