Abstract
In this study, we prepared chitosan (CS)-coated iron oxide (Fe3O4) nanocomposites (NCs) by employing the aqueous leaf extract of Brassica oleracea L. and evaluated its antimicrobial potential. The characterization of hybrid CS-Fe3O4 NCs was performed using Fourier-transform infrared spectroscopy (FTIR) analysis to evaluate the chemical bonding of chitosan to nanoparticles (NPs). X-ray photoelectron spectroscopy (XPS) studies revealed the presence of oxidation state elements Fe 2p, O 1s, N 1s, and C 1s, and the zeta potential analysis was found to have well-colloidal stability (+ 76.9 mV) of NCs. Transmission electron microscopy (TEM) analysis determined that CS-Fe3O4 NCs were spherical with an average particle size of 27 nm. The X-ray diffractometer (XRD) spectrum ascertained the crystallinity of the hybrid NCs and the vibrating sample magnetometer (VSM) inferred the ferromagnetic behavior of the synthesized NCs. Furthermore, the significant antibacterial efficacy of NPs was demonstrated against foodborne bacterial pathogens, such as Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli), and the highest zone of inhibition was observed to be 11.5 mm and 13.5 mm in CS-Fe3O4 NCs, respectively. In comparison with Fe3O4 NPs, synergistic impacts of CS-Fe3O4 NCs displayed great antibacterial potential as exhibited by a clearly enlarged zone. Thus, CS-Fe3O4 NCs could be used as efficacious antimicrobial agents in food packaging and food preservation fields.
Keywords: Antibacterial activity, Brassica oleracea, Chitosan-coated Fe3O4 nanocomposite, Colloidal stability, Ferromagnetic, Surface modification
Introduction
Nanotechnology is progressing forward rapidly in recent years, mainly the development of metal oxide coated with biopolymer nanocomposites has drawn significant attention in food and biomedical fields. Several nanomaterials, notably iron oxide (Fe3O4) nanoparticles (NPs), have been widely applied in biomedicine (Andrade et al. 2020; Ahmadi et al. 2021), food preservation (Thirumurugan et al. 2020), catalyst (Vasantharaj et al. 2019), cosmetics (Ana et al. 2014), and clinical fields (Kouhbanani et al. 2019). Besides these, Fe3O4 NPs are even used as effective antimicrobial agents (Jagathesan and Rajiv 2018; Bharathi et al. 2019; Bhuiyan et al. 2020) due to their significant variable oxidation states with magnetic, biocompatible, nontoxic, and cost-effective properties (Nehra et al. 2018; Powell et al. 2020). Although numerous physical and chemical methods have been developed for the preparation of iron oxide NPs, they are expensive, and toxic chemicals are used as reducing and stabilizing agents causing public health and environmental problems. Therefore, the development of novel green routes for the iron oxide NPs synthesis is very timely and important.
Surface modification of metal oxide NPs with phytocompounds can enhance their physico-chemical and biological properties. Amino acids, carbohydrates, phenol, sugar, and proteins in plant materials have been found to possess reducing and stabilizing function in the synthesis of metal oxide NPs (Kumar et al. 2014; Sivaraj et al. 2014). In recent times, plant-based NPs are increasingly gaining attention because of their cost-effective, safe, and nontoxic properties. For instance, several iron oxide NPs synthesized using phytocompounds of Ruellia tuberosa (Vasantharaj et al. 2019), Terminalia bellirica, and Moringa oleifera (Jegadeesan et al. 2019), Centella asiatica (Poka et al. 2019), Nephrolepis auriculate (Yi et al. 2019), Rhamnus triquetra (Abbasi et al. 2020), Pheonix dactylifera (Abdullah et al. 2020), and Thunbergia grandiflora (Pai et al. 2021) have been reported.
A positive association has been found between phytochemicals and chronic diseases. Broccoli, one of the cruciferous vegetables, is associated with many health benefits and is intensively consumed. The residue of broccoli (leaves) contains several high-quality compounds, such as sulforaphane, phenolic bioactive compounds, and protein (Campas-Baypoli et al. 2010; Osuntokun et al. 2019). Anita and Prakash (2013) have reported significant antibacterial property in silver NPs prepared from broccoli.
Fe3O4 NPs surface may be coated with various organic or inorganic compounds (surfactants, polymers, peptides, etc.), which tends to make these NPs more stable and biocompatible, and prevents their oxidation. In this case, a biopolymer chitosan (CS), a natural polycationic polymer with reactive functional groups, has been used as a reducing and antimicrobial agent (Manikandan and Sathiyabama 2016; Shanmuganathan et al. 2019; Ying et al. 2020). Chitosan has been frequently used as a surface coating for metal NPs owing to its attractive physicochemical and biological properties (Ottonelli et al. 2020). The amino groups of chitosan are quickly protonated in the acidic condition and are capable of binding to anionic functional groups. For high-affinity hybridization, large amounts of amino groups on the chitosan play a critical role. It seems that the abovementioned processes result in easy binding of iron oxide NPs with an oxygen-containing functional group between amino groups of chitosan (Zhang et al. 2018).
Earlier attempts on the synthesis of metal oxide NPs using chitosan as a reductant play a vital role in biomedical field (Manikandan and Sathiyabama 2015; Ma et al. 2020; Nguyen et al. 2020; Varukattu et al. 2020). Recently, chitosan-coated iron oxide NCs was demonstrated to possess antibacterial and antioxidant activity (Nehra et al. 2018; Bharathi et al. 2019), antituberculosis activity (El Zowalaty et al. 2015), magnetite property (Pham et al. 2016), and environmental remediation applications (Kakavandi et al. 2019; Noorisepehr et al. 2020). The antifungal activity of iron oxide NPs functionalized with chitosan was examined against Candida albicans and Candida glabrata biofilms (Arias et al. 2020). As reported by Qu et al. (2013), chitosan-coated Fe3O4 NPs were formulated with the coupling of PEG (polyethylene glycol) chains to investigate antitumor studies. Rezaei et al. (2019) observed the efficient degradation of pesticides using PMS (peroxymonosulfate) decomposition by a hybrid system of zinc oxide NPs decorated on magnetic core/shell structure (ZnO/SiO2/Fe3O4).
To date, there are no scientific reports about the synthesis of chitosan-coated iron oxide nanocomposites using leaf extract of Brassica oleracea L. in a nonhazardous way to the best of our knowledge. Therefore, the aim of our study is green synthesis of chitosan-coated iron oxide nanocomposites (CS-Fe3O4 NCs) using broccoli leaf extracts as reducing agents. This has been developed using the morphology, magnetic properties, and characteristic of prepared hybrid nanocomposites by dynamic light scattering (DLS), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), vibrating sample magnetometer (VSM), X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), and X-ray diffractometer (XRD), etc., and their antibacterial activities against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were further studied.
Materials and methods
Materials
Iron (III) chloride hexahydrate (FeCl3.6H2O) was acquired from Aladdin Industrial Corporation (Shanghai, China). Chitosan (91% deacetylation) was obtained from Qingdao Honghai Biotechnology Company (Shandong, China). Nutrient broth (NB) and luria broth (LB) were supplied by Hangzhou Microbial Reagent Co., Ltd. (Zhejiang, China). Foodborne pathogenic bacterial strains of E.coli and (CICC 21524) and S. aureus (CICC 10384) were purchased from China Center of Industrial Culture Collection (Beijing, China).
Preparation of aqueous leaf extracts
Fresh broccoli (Brassica oleracea L.) was purchased from the commercial market in Hangzhou, China. After the removal of adherent surface debris with distilled water, broccoli leaves were chopped into small pieces and dried at room temperature. They were then ground by a mixing grinder, and 2 g of resulting fine powder was mixed with double-distilled water (100 mL) and stirred at 80 °C for 30 min. The mixture was then filtered, and the filtrate was stored at 4 °C for nanoparticles preparation.
Synthesis of iron oxide nanoparticles using broccoli aqueous extracts
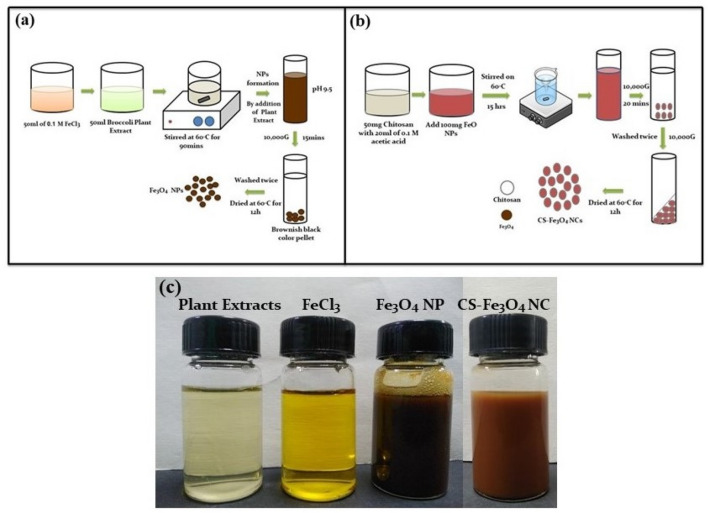
Typically, 1.35 g of FeCl3.6H2O was dissolved in double-distilled water (50 mL) to provide aqueous FeCl3 solution (0.1 M), and 50 mL of broccoli aqueous extract and 50 mL of 0.1 M FeCl3 solution were mixed and stirred at 80 °C for 90 min. The pH of the mixture was adjusted to 9.5 using 0.2 M sodium hydroxide (NaOH). The formation of metallic Fe3O4 nanostructure was clearly visible through the color change from yellowish to brownish-black, indicating that FeCl3 was reduced by broccoli aqueous extract. The resulting precipitate was collected by centrifugation (Fig. 1a) and subsequently dried at 60 °C for 12 h to provide 117 mg of Fe3O4 NPs.
Fig. 1.
Schematic illustration of the biosynthesis of a Fe3O4 NPs using extract of broccoli leaf, b CS-Fe3O4 nanocomposites. Colorization photographs c of synthesized Fe3O4 NPs and CS-Fe3O4 NCs
Preparation of chitosan-coated Fe3O4 nanocomposites
Generally, 100 mg of prepared Fe3O4 NPs and 20 mL of chitosan (50 mg of chitosan in 20 mL of 0.1 M acetic acid) solution were mixed and stirred at 60 °C for 15 h. Along with the reaction process, the color of mixture was changed from brownish-black to brown. After centrifugation (10,000g, 20 min), the pellets were collected, washed with double-distilled water (Fig. 1b), and dried in a hot air oven at 60 °C to provide 75 mg of CS-Fe3O4 NCs.
Characterization techniques
After complete removal of excess unreacted debris, the obtained nanoparticles were well characterized.
Spectroscopic studies of nanoparticles
To perform optical wavelength UV–vis (Ultraviolet–visible), aliquot nanoparticles were dissolved in distilled water, and the UV–vis spectra were recorded with a UV-3600 spectrophotometer (Shimadzu, Japan) at room temperature. FTIR spectra of chitosan, leaf extract, and prepared nanoparticles were analyzed with a Nicolet 380 spectrometer (Thermo, USA). The surface speciation, oxidation state, and the element existence of the nanoparticles were characterized by XPS, and the study was conducted by Thermo Fisher K-Alpha, USA.
Electron microscopic studies of nanoparticles
Energy-dispersive spectroscopy (EDS) (Bruker Nano Berlin, Germany) is an analytical method used to monitor the elemental percentage analysis of the nanoparticles while obtaining along with FESEM (ZEISS Sigma 500). TEM (JEOL JEM-2100 F, Japan) was used to structurally characterize prepared NPs. The magnetic behavior of prepared NPs was evaluated with vibrating sample magnetometer (VSM) (JC08-HH15, Nanjing, China) at room temperature.
Dynamic light scattering and X-ray diffractometer studies of nanoparticles
The average particle size and zeta potential of the prepared nanoparticles were measured by dynamic light scattering (DLS) with a Zetasizer Nano ZS (Malvern, UK). The crystalline structure of the NPs was examined on an XRD (PANalytical Empyrean, Netherlands).
Antibacterial activity assessment
The antibacterial activity assay was conducted by disc diffusion techniques. The bacterial strains (E. coli and S. aureus) were incubated on the nutrient broth at 37 °C for 12 h. In the activity experiment, 50 µL of 1 × 106 bacterial suspensions were swabbed on the Mueller–Hinton Agar (MHA) plates and allowed to dry for 30 min. Then, the 5 mm sterile discs were gently placed on the dried plates and added different concentrations of nanoparticles (50, 100, 150, and 200 µg/mL). The pure chitosan is used as a control. All plates were incubated at 37 °C for 24 h, followed by the measurement of the inhibition zone.
Statistical analysis
All the data were subjected to one-way analysis of variance to determine the significance of individual differences at p < 0.01 and 0.05 levels. All statistical analyses were conducted using SPSS 16.0 (2008) software support.
Results and discussion
Various techniques of the iron oxide nanoparticle synthesis have been developed; however, a synthesis strategy of NPs with the uniform size distribution and small particles, also with good stability to preclude agglomeration, is an ongoing exploration. Herein, we focused on a green approach to prepare iron oxide nanoparticles using broccoli leaf extract and chitosan. The synthesis procedure is illustrated in Fig. 1.
The formation of Fe3O4 NPs by the reductant of FeCl3 with broccoli solution is visually inspected by yellowish to brownish-black precipitate (Fig. 1c). Similar color change in the synthesis of iron oxide nanoparticles using plant materials as reductant agents has been reported (Mirza et al. 2018; Poka et al. 2019; Vasantharaj et al. 2019). As shown in Fig. 1c, after chitosan solution addition, the color of the mixture changed from brownish-black to brownish color, indicating the formation of CS-Fe3O4 NCs, which was in agreement with a previous report (Nehra et al. 2018).
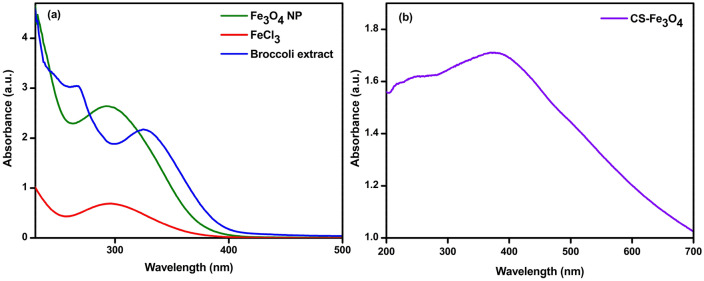
UV–vis analysis is useful in the characterization of NPs and provides certain information regarding the stability, shape, and size of the NPs (Deshmukh et al. 2019). The UV–vis spectra of Fe3O4 NPs and CS-Fe3O4 NCs are presented in Fig. 2a and b. The strong surface plasmon resonance (SPR) centered at 290 and 324 nm were attributed to Fe3O4 NPs and CS-Fe3O4 NCs, respectively. These findings are in harmony with Ahmadi et al. (2021) who investigated green synthesis of FeO NPs from Satureja hortensis essential oil and observed UV–vis spectra between 250 and 350 nm. Samrot et al. (2018) also indentified SPR peaks at 225 and 360 nm in the uncoated and chitosan-coated iron oxide nanoparticles, respectively. A considerable amplification of intensity which is specific for the Fe3O4 NPs and CS-Fe3O4 NCs could be observed.
Fig. 2.
UV-absorbance spectrum of prepared a Fe3O4 NPs and b CS-Fe3O4 NCs
FTIR spectrum of broccoli plant extract, chitosan, synthesized Fe3O4 NPs, and CS-Fe3O4 NCs are shown in Fig. 3. In the spectrum of broccoli plant extracts, a broad spectrum at 3357 cm−1 is assigned to O–H stretching of the hydroxyl group of phenols. The peak at 2875 cm−1 has corresponded to the C–H symmetric and asymmetric of the aliphatic group. While the peaks at 1617, 1381, 1031 and 547 cm−1 have been attributed to N–H stretching vibration, C–Br (alkyl halides) stretching, and C–O (alcohol group) stretching vibration, respectively (Fig. 3d). According to the literature, all of the absorption bands of broccoli leaf extracts were characterized (Osuntokun et al. 2019). Narrower absorption peaks at 535 and 422 cm−1 of Fe3O4 NPs are supposed owing to the presence of metal–oxygen (Fe–O), which matched with several studies (Amutha and Sridhar 2018; Abdullah et al. 2020; Aziz 2020). The peaks at 3310 cm−1 and 2914 cm−1 are the characteristic stretching vibration attributed to O–H and C–H from terpenoids, flavonoids, and proteins (Fig. 3b), while the N–H stretching vibration (1617 cm−1) appeared in Fig. 3d shifted to 1590 cm−1 (Fig. 3b), confirming the integration of the biomolecules with the synthesized Fe3O4 NPs (Mott et al. 2010). In prior FTIR study of Fe3O4-NPs synthesized by Tie Guany in tea extract, identified functional groups are present (Xin et al. 2016). The formation of CS-Fe3O4 NCs was confirmed by FTIR spectra. As shown in Fig. 3a and c, the characteristic shoulder peaks of chitosan at 1589 cm−1 and 1578 cm−1, corresponding to the stretching peaks of the amide C=O bonds (Fig. 3c), shifted to 1628 cm−1 which is due to the covalent bond that is established between the amino groups of chitosan molecules and propyl-(1H-tetrazole-5-yl) amine-trimethoxysilane (Kashkouli et al. 2018). The presence of two strong bands at 593 and 443 cm−1 revealed the metal–oxygen (Fe–O) (Fig. 3a). Based on the result of FTIR, the functional groups of broccoli can be used as a reducing agent for the formation of Fe3O4 NPs and the chelation of iron with the presence of O–H and N–H of chitosan.
Fig. 3.
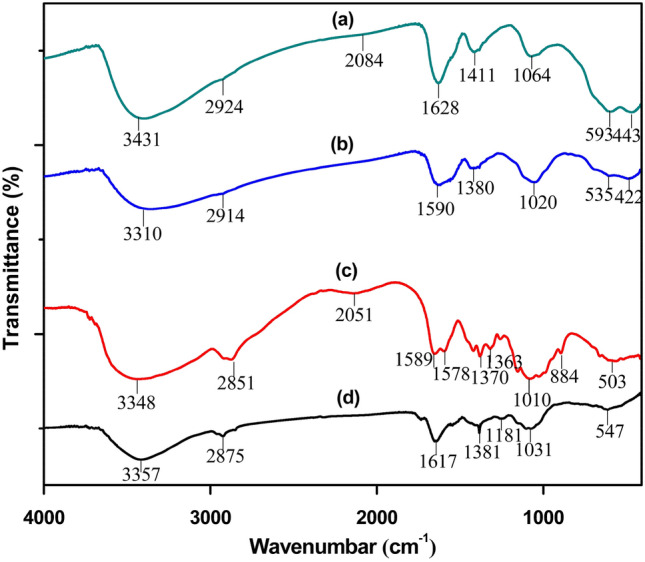
FTIR spectra of prepared a CS-Fe3O4 NCs, b Fe3O4 NPs, c pure chitosan and d broccoli leaf extract were assessed in % transmittance within the wavenumber frequency range 4000–400 cm−1
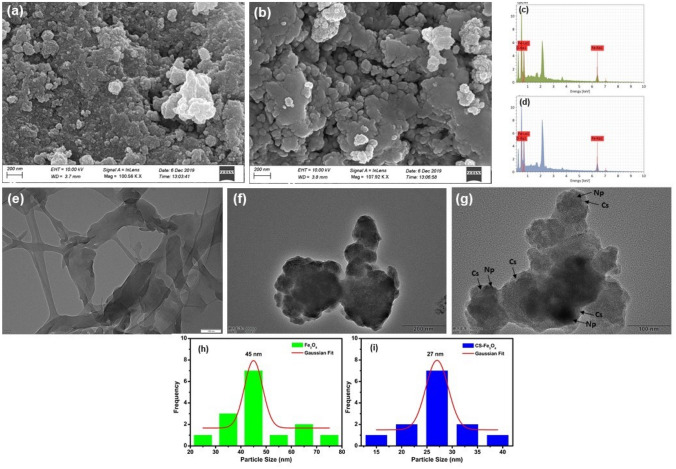
Morphology of synthesized Fe3O4 NPs and CS-Fe3O4 NCs was determined by FESEM, and the composition of elements were investigated by EDS. The spherical-shaped particles with agglomeration were found in both prepared CS-Fe3O4 NCs (Fig. 4b) and Fe3O4 NPs (Fig. 4a) by FESEM, in which it can be shown that bare Fe3O4 NPs is nanometer scale and has the smallest size distribution. The size of particle increases during the chitosan coating process, as the size of the individual particle is correlated to the thickness of the chitosan layer (Podrepšek et al. 2020). The presence of biological compounds on the surface of the particles can cause agglomeration. TEM analysis was employed for further evaluation of shape and measuring size of prepared NPs. TEM micrograph revealed that the prepared Fe3O4 NPs and CS-Fe3O4 NCs were spherical with an average particle size of 45 and 27 nm, respectively (Fig. 4g and i), also clearly displaying the encapsulation of chitosan shell-coated Fe3O4 NPs (Fig. 4g). Similar sizes of spherical Fe3O4 NPs synthesized from various plant sources such as Cynara cardunculus (Ruíz-Baltazar et al. 2019), gram bean (Koli et al. 2019), Juglans regia green husk (Izadiyan et al. 2018) were previously reported by TEM analysis. In addition, Azizi (2020) noticed that Fe3O4/cellulose nanocomposite (15.5 nm) has a smaller size than pure Fe3O4 NPs (28 nm). As shown in the EDS spectrum (Fig. 4c), Fe and O are the major compositions of prepared Fe3O4 NPs. The presence of Fe and O in the Fe3O4 NPs indicate residual biomolecules on Fe3O4 NPs surface. The high percentage of Fe and O elements was traced in the biosynthesized FeO NPs (Jegadeesan et al. 2019; Poka et al. 2019; Bhuiyan et al. 2020). Similar results were observed by Pham et al. (2016) who have examined the shape of prepared Fe3O4 NPs as well as CS-Fe3O4 NCs with a diameter range from 8 to 20 nm. EDS spectrum of CS-Fe3O4 NCs showed the presence of Fe, C, and O compositions revealing that chitosan was coated with Fe3O4 NPs (Fig. 4d).
Fig. 4.
FESEM micrograph of a synthesized Fe3O4 NPs, b CS-Fe3O4 NCs. All the scale bars are 200 nm. EDS spectrum of c Fe3O4 NPs and d CS-Fe3O4 NCs. Bright-field TEM image and size distribution of f, h Fe3O4 NPs, g, i CS-Fe3O4 NCs and e pure chitosan
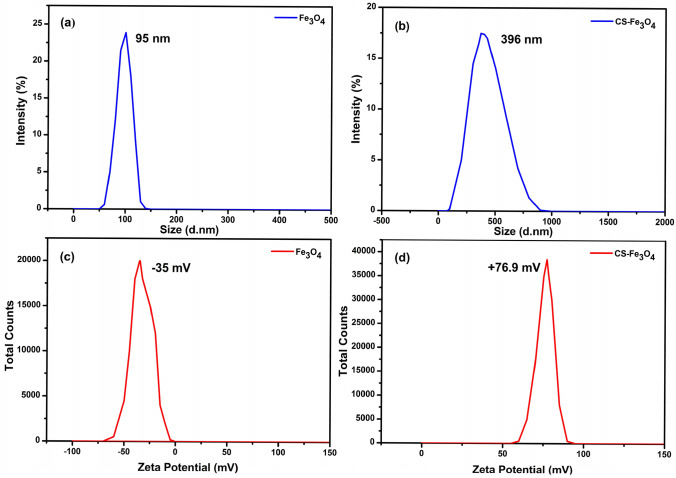
The average size of synthesized Fe3O4 NPs was around 95 nm, and the negative surface charge of zeta potential was − 35 mV (Fig. 5a and c), implying that their strong negative surface charge causes the synthesized NPs highly stable (Kanagasubbulakshmi and Kadirvelu. 2017), which means the obtained findings are in agreement with a previous study (Ahmadi et al. 2021). A significant increase in particulate surface charge assumed small size and good stability when the biomolecules bonded to the surface of Fe3O4 (Muthukumar and Manickam 2015). After the addition of chitosan onto Fe3O4 NPs, the average particle size and zeta potential was 396 nm, and the positive surface charge was + 76.9 mV (Fig. 5b and d). Under the experimental condition, chitosan is positively charged because of the protonation of free amine groups on the surface of nanocomposite (Zamora-Mora et al. 2014; Castelló et al. 2015). In addition, it has an immoderate adhesiveness for negatively charged surfaces. Several investigators have reported that synthesis of chitosan-FeO nanocomposites with the positive surface charge of zeta potential (Arakha et al. 2015; Qiu et al. 2021). The reversal of surface potential is correlated with XRD analysis and SPR spectra of CS-Fe3O4 NCs.
Fig. 5.
Dynamic light scattering depicting the particle size and zeta potential of Fe3O4 NPs (a, c) and CS-Fe3O4 NCs (b, d), respectively. The measurement of synthesized NPs was performed in deionized water media
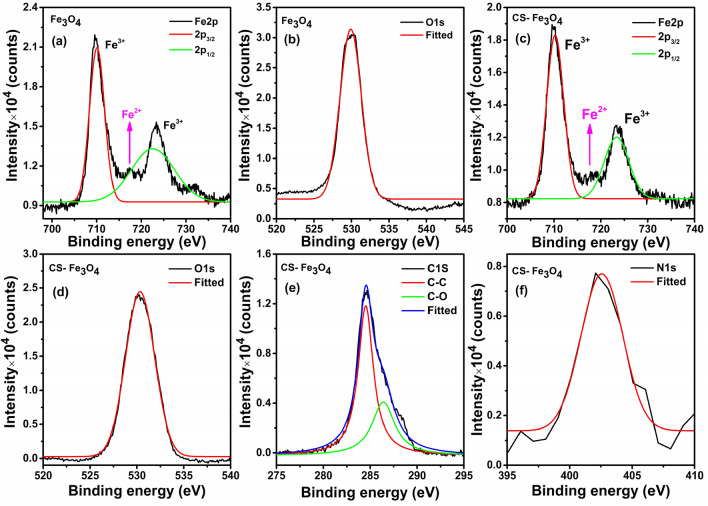
XPS is a potential technique to confirm the composition and valance state of the Fe3O4 NPs surface (Gao et al. 2020). The wide survey spectra of Fe3O4 NPs and CS-Fe3O4 NCs confirmed the existence of Fe 2p, O 1s, C 1s, and N 1s elements. As illustrated in Fig. 6a, Fe 2p spectrum of FeO is resolved into two major photoelectron peaks at 710.07 eV and 722.58 eV attributed to Fe2p3/2, Fe2p1/2, respectively (Alaghmandfard and Hosseini 2021; Pai et al. 2021), whereas the energy of CS-Fe3O4 (Fig. 6c) is slightly higher at 710.24 eV and 723.35 eV, respectively. The increment of peaks is supposed to be Fe3+ ions along with a tiny peak at ~ 717 eV of Fe2+ ions (Gao et al. 2020). Likewise, O 1s spectrum (Fig. 6d) of CS-Fe3O4 NCs demonstrates the higher binding energy (530.34 eV) (AbuTalib et al. 2021) compared to that of Fe3O4 NPs (529.90 eV) (Fig. 6b). XPS spectra of chitosan associated with Fe3O4 NPs are shown in Fig. 6e and f. The high-resolution spectrum of CS-Fe3O4 NCs (Fig. 6e) illustrates two shoulders at 284.52 eV and 286.40 eV associated with C–C and C–O bonds, respectively (Yi et al. 2019; Karthika et al. 2020), and binding energy at 402.8 eV (Fig. 6f) ensures the contribution of high protonated amine (–NH3+) groups involved in hydrogen bonding owing to N 1s core level (Lawrie et al. 2007; Yu et al. 2017; Zhang et al. 2018), indicating that Fe3O4 NPs successfully coated with chitosan. A series of experimental results previously reported values and the list of appropriate C 1s, O 1s, and N 1s binding energy (BE) for different nanomaterials (Srihasam et al. 2020; Liao et al. 2021; Sudha et al. 2021). In view of the XPS spectra, it can be concluded that the prepared CS-Fe3O4 NCs mixed Fe ions (3+ and 2+) was successfully coated with chitosan.
Fig. 6.
XPS high-resolution pattern of bare Fe3O4 NPs a Fe 2p spectra, b O 1s spectra and CS-Fe3O4 NCs, c Fe 2p spectra, d O 1s spectra, e C 1s spectra and f N 1s spectra
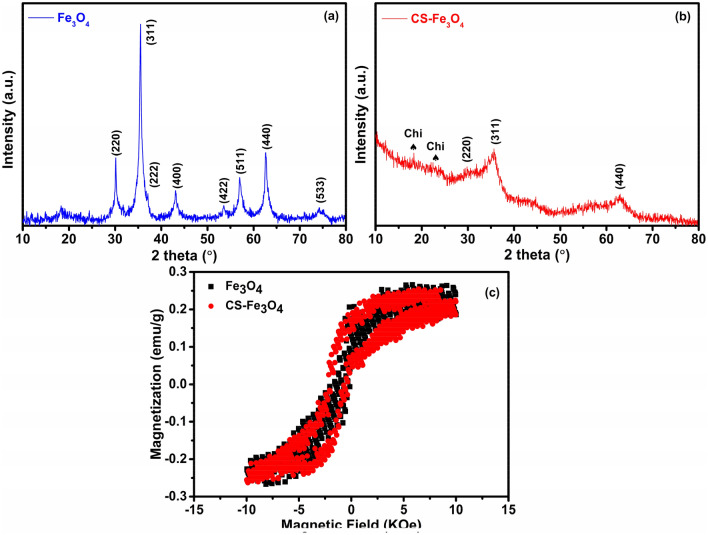
XRD patterns of Fe3O4 NPs and CS-Fe3O4 NCs are shown in Fig. 7a and b. For Fe3O4 NPs, the major diffraction peaks at 2θ = 30°, 36°, 37°, 43°, 54°, 57°, 63°, and 74° correspond to the characteristic face-centered cubic (FCC) structure of iron lines indexed at (220), (311), (222), (400), (422), (511), (440), and (533), respectively, indicative of the crystalline nature (Fig. 7a). These findings are in line with those previously published (Pérez et al. 2020; Sorasitthiyanukarn et al. 2020; Dhavale et al. 2021). The configuration and relative intensity of all peaks confirm the spatial structure of Fe3O4 and demonstrate that the NPs are constituted of magnetite-Fe3O4 and do not include the impurity phase of goethite α-FeO(OH) and hematite (Fe2O3), reflecting diffraction peaks of (110), (104) at 2θ values of 21°, 33°, respectively (Arakha et al. 2015; Pham et al. 2016; Asab et al. 2020). In addition, clear evidence to identify Fe3O4 but not Fe2O3 is the XRD peak position of major (311) plane, corresponding to a higher 2θ value (35.5°) specified to be Fe3O4, while that of Fe2O3 is located at 33.2° (Noval and Carriazo 2019). Moreover, Fe3O4 shows a tiny hump at 37.16° corresponding to (222) plane, which should not be observed in the case of Fe2O3 according to the literature (Muthukumar and Manickam 2015; Ruíz-Baltazar et al. 2015). Overall, the diffractogram matches well with the standard XRD pattern of Fe3O4 (JCPDS card no. 89-0691). According to the literature (Safari and Javadian 2014), the presence of chitosan in the XRD pattern was clearly stated as very weak broad band at 2θ 12°–20° appeared in the Fe3O4-chitosan MNPs, which could be related to the amorphous chitosan formed around the Fe3O4 NPs; therefore, the diffraction angles at 18° and 23° shown in the CS-Fe3O4 NCs pattern are due to the crystal structure of chitosan (Fig. 7b) (Unsoy et al. 2012; Kashkouli et al. 2018; Ayyanaar et al. 2020). Furthermore, the disappearance of diffraction peaks at 2θ = 43°, 54°, 57°, and 74°, and the decreased intensity of other diffraction lines suggest that the Fe3O4 NPs were effectively coated by chitosan (Fig. 7b) (Salah El-Din et al. 2011; Safari and Javadian 2014). The crystallite size of the NPs was calculated by Debye Scherrer formula. According to D = kλ/β cos θ, whereas k is the Scherrer constant (0.9), λ is the incident X-ray wavelength (nm), β is the full width at half-maxima of the peak and θ is the corresponding Bragg’s angle (Cuong et al. 2012), the crystallite sizes of Fe3O4 NPs and CS-Fe3O4 NCs were calculated to be around 14 and 3 nm, respectively. Furthermore, the Lu et al. (2019) study recorded a decrease in CS-Fe3O4 nanocomposite particle size compared to bare nanoparticles, as nanoparticles are polymerized and bonds are formed to prevent nanoparticles and eventually nanocomposites from increasing.
Fig. 7.
Representative XRD pattern of a Fe3O4 NPs, b CS-Fe3O4 NCs. The characteristic peak of chitosan is labeled with Chi♠. Magnetic behavior of c pristine Fe3O4 NPs and CS-Fe3O4 NCs measured by VSM, showing decreased magnetization after chitosan coating
Magnetic hysteresis curves of Fe3O4 NPs and CS-Fe3O4 NCs at room temperature are shown in Fig. 7c. Magnetization hysteresis was obtained by modifying H between Oe + 10,000 to ‒10,000 Oe. The saturation magnetization of (Ms) Fe3O4 NPs and CS-Fe3O4 NCs were 0.265 and 0.253 emu/g, respectively. The lower value of the measured saturated magnetization, indicative of the small size magnetite and diminished saturated magnetization values in CS-Fe3O4 NCs, attributes to the chitosan polymer incorporated with Fe3O4 NPs. This phenomenon proved the ferromagnetic behavior of synthesized magnetic nanomaterials (Konwar et al. 2015, 2016). Similar findings were also reported in the literature (Badry et al. 2017; Kothavale et al. 2019; Suo et al. 2019) regarding the magnetization values of prepared CS-Fe3O4 NCs decreased, by comparison with that of Fe3O4 NPs, which is due to the magnetite incorporation in the chitosan coating.
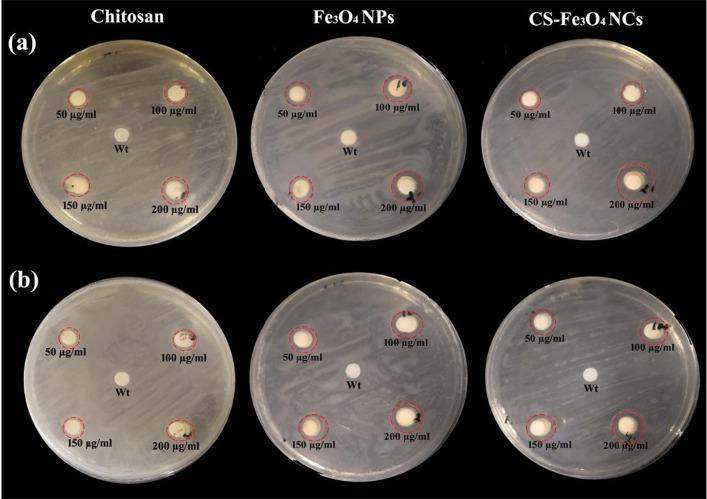
The antibacterial activities of Fe3O4 NPs and CS-Fe3O4 NCs against Gram-positive S. aureus and Gram-negative E. coli strains were further examined using disc diffusion techniques. The diameter of the zone of inhibition against test strains is shown in (Table 1, Fig. 8). The zone of inhibition of CS-Fe3O4 NCs is extensively increased as compared to Fe3O4 NPs and chitosan alone. In CS-Fe3O4 NCs (200 µg/mL), the highest zone of inhibition was observed 13.5 mm for E. coli and 11.5 mm for S. aureus compared to those in Fe3O4 NPs and chitosan. The obtained outcomes demonstrate that CS-Fe3O4 NCs have a powerful development growth inhibition zone of 17.4% and 9.5% for E.coli and S. aureus, respectively, compared to those in Fe3O4NPs. The zone of inhibition due to bacterial cell wall lysis or metal ions which cause bacterial DNA damage (Behera et al. 2012; Mirza et al. 2018). Moreover, because of the surface modification of Fe3O4 NPs, effective attractive interaction at interface may result in more toxicity to bacterial cells, while less antibacterial activity has been found in bare Fe3O4 NPs. A positively charged chitosan interacts with negatively charged cell membranes, leading to increased cell permeability and lysis of the membrane. Chitosan also restricts the production of bacteria by the chelation of essential metals and nutrients. The current investigation is in close agreement with previous literature as chitosan-coated cobalt ferrite (CoFe2O4) NPs revealed good antibacterial activity against Gram-negative (Pseudomonas aeruginosa and E. coli) and Gram-positive strains (Enterococcus faecalis and S. aureus) (Gingasu et al. 2018). Similar findings were also obtained from chitosan-coated Fe3O4 NPs as well as Fe3O4 NPs, showing inhibition against E. coli (Maria Dhivya et al. 2016). The antibacterial activity of both nanomaterials displayed lower growth inhibition zone on Gram-positive bacteria than on Gram-negative bacteria. This might be because of the differences within the cell wall structure of Gram-positive and Gram-negative bacteria. Kalaivani et al. (2018) reported that chitosan-mediated silver NPs exhibited highly potent antibacterial activity against Gram-negative bacterial strains Pseudomonas sp. and E. coli than Gram-positive strains Bacillus sp. and Staphylococcus sp. Shrifian-Esfahni et al. (2015) demonstrated that positively charged amino groups of chitosan interact with negatively charged components of bacterial cell wall such as neuraminic acid and N-acetylmuramic acid, and the resulting chitosan can contribute to bacterial suppression by chelating various transitional metal ions. Furthermore, intracellular reactive oxygen species (ROS) production may be a factor in the antibacterial effect of NPs in pathogen cells. Previous findings have documented that ROS also play an important role in cell signaling, which includes apoptosis, gene expression, and the activation of cell signaling cascades (Hancock et al. 2001). The effective bactericidal activity was demonstrated due to the generation of ROS by silver NPs (Park et al. 2009). Hence, the microbial growth inhibition of CS-Fe3O4 NCs is supposed to be through the generation of ROS. Further studies will be carried out on the antibacterial mechanisms and antifungal activity.
Table 1.
The diameter of bacterial growth inhibition (mm) of chitosan, Fe3O4 NPs and CS-Fe3O4 NCs against test strains
| Samples | Concentrations (µg/ml) | Zone of inhibition (mm) Diameter | |
|---|---|---|---|
| E. coli | S. aureus | ||
| Chitosan | 50 | 6.9 ± 0.2c | 6.7 ± 0.1c |
| 100 | 7.2 ± 0.1b | 7.2 ± 0.2a | |
| 150 | 6.9 ± 0.3c,d | 6.5 ± 0.2d | |
| 200 | 7.5 ± 0.1a | 6.9 ± 0.3b | |
| Fe3O4 | 50 | 9.0 ± 0.5d | 9.0 ± 0.3d |
| 100 | 10.5 ± 0.5c | 9.5 ± 0.2c | |
| 150 | 11.0 ± 0.3b | 10.0 ± 0.3b | |
| 200 | 11.5 ± 0.5a | 10.5 ± 0.5a | |
| CS-Fe3O4 | 50 | 9.5 ± 0.3d | 8.5 ± 0.3d |
| 100 | 10.0 ± 0.2c | 8.6 ± 0.2c | |
| 150 | 10.2 ± 0.2b | 10.1 ± 0.5b | |
| 200 | 13.5 ± 0.5a | 11.5 ± 0.5a | |
Mean values followed by different letters within a column are significantly different (p < 0.05, by one-way ANOVA and Duncan’s multiple range test using SPSS 16.0, 2008). Whereas (a) represents high, (b) less, (c) very less, (d) no significant levels and (cd) do not differ significantly between c and d
Fig. 8.
Agar diffusion assay for measuring the antibacterial activity of Fe3O4 NPs and CS-Fe3O4 NCs at 50, 100, 150 and 200 µg/mL concentrations with distilled H2O (Wt), pure chitosan used as control against a E. coli and b S. aureus
Conclusions
It can be safe concluded that a simple, economic, and eco-friendly approach for preparing uncoated and chitosan-coated Fe3O4 NPs using leaf extracts of broccoli has been developed, and the distinctive characteristics of these NPs have been discussed in a comprehensive manner. The synthesis of iron oxide NPs, functionalized with chitosan, was completely successful. It should be noted that the zeta potential was greater than 30 mV for NPs, which ensured strong electrostatic stabilization, and a smaller diameter with narrow size distribution, and ferromagnetism behavior were confirmed by TEM and VSM, respectively. Most importantly, the CS-Fe3O4 NCs exhibited potentially higher antibacterial activity against E. coli and S. aureus strains, inferring that NCs can be used not only as a potential antibacterial agent against foodborne pathogenic bacteria but also for the development of drug delivery in near future.
Acknowledgements
We thank the Natural Science Foundation of Zhejiang Province of China (2017C32008), and the Natural Science Foundation of Zhejiang Province (LY20B040001).
Author contributions
MA: conceptualization, methodology, software, investigation, writing-original draft preparation, ZL: formal analysis, investigation, DZ: methodology, investigation, JH: conceptualization, supervision, visualization, writing—review and editing.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Abbasi BA, Iqbal J, Zahra SA, Shahbaz A, Kanwal S, Rabbani A, Mahmood T (2020) Bioinspired synthesis and activity characterization of iron oxide nanoparticles made using Rhamnus Triquetra leaf extract. Mater Res Express 6:1250e7. 10.1088/2053-1591/ab664d/meta
- Abdullah AA, Eddine LS, Abderrhmane B, Gonzalez A, Guerrero A, Romero A. Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain Chem Pharm. 2020;17:100280. doi: 10.1016/j.scp.2020.100280. [DOI] [Google Scholar]
- AbuTalib NH, LaGrow AP, Besenhard MO, Bondarchuk O, Sergides A, Famiani S, Ferreira LP, M. Cruz MM, Gavriilidis A, Thanh NTK (2021) Shape controlled iron oxide nanoparticles: Inducing branching and controlling particle crystallinity. Cryst Eng Commun 23:550–561. 10.1039/d0ce01291b
- Ahmadi S, Fazilati M, Nazem H, Mousavi SM. Green synthesis of magnetic nanoparticles using Satureja hortensis essential oil toward superior antibacterial/fungal and anticancer performance. BioMed Res Int. 2021 doi: 10.1155/2021/8822645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaghmandfard A, Hosseini HRM. A facile, two-step synthesis and characterization of Fe3O4–LCysteine–graphene quantum dots as a multifunctional nanocomposite. Appl Nanosci. 2021;11:849–860. doi: 10.1007/s13204-020-01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amutha S, Sridhar S. Green synthesis of magnetic iron oxide nanoparticle using leaves of glycosmis mauritiana and their antibacterial activity against human pathogens. J Innov Pharma Biol Sci. 2018;5:22–26. [Google Scholar]
- Ana LS, Riansares MO, Jon SL, Carmen C. Nanoparticles: A global vision. Characterization, separation, and quantification methods Potential environmental and health impact. Anal Methods. 2014;6:38–56. doi: 10.1039/C3AY40517F. [DOI] [Google Scholar]
- Andrade RGD, Veloso SRS, Castanheira EMS. Shape anisotropic iron oxide-based magnetic nanoparticles: synthesis and biomedical applications. Int J Mol Sci. 2020;21:2455. doi: 10.3390/ijms21072455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anita M, Prakash P. Combined antibacterial activity of antibiotics and biosynthesized silver nanoparticles from broccoli (Brassica oleracea) Int J Univ Pharm Biosci. 2013;2:146–158. [Google Scholar]
- Arakha M, Pal S, Samantarrai D, Panigrahi TK, Mallick BC, Pramanik K, Mallick B, Jha S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci Rep. 2015;5:14813. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias LS, Pessana JP, Netoa FNS, Limab BHR, Camargoc ER, Ramaged G, Delbema ACB, Monteiro DR. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf B. 2020;192:111080. doi: 10.1016/j.colsurfb.2020.111080. [DOI] [PubMed] [Google Scholar]
- Asab G, Zereffa EA, Seghne TA. Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: characterization and evaluation of antimicrobial activity. Int J Biomater. 2020 doi: 10.1155/2020/4783612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyanaar S, Balachandran C, Bhaskar RC, Kesavan MP, Aoki S, Raja RP, Rajesh J, Webster TJ, Rajagopal G. ROS-responsive chitosan coated magnetic iron oxide nanoparticles as potential vehicles for targeted drug delivery in cancer therapy. Int J Nanomed. 2020;15:3333–3346. doi: 10.2147/IJN.S249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi A. Green synthesis of Fe3O4 nanoparticles and its application in preparation of Fe3O4/cellulose magnetic nanocomposite: a suitable proposal for drug delivery systems. J Inorg Organomet Polym Mater. 2020 doi: 10.1007/s10904-020-01500-1. [DOI] [Google Scholar]
- Badry MD, Wahba MA, Khaled R, Ali MM, Farghali AA. Synthesis, characterization and in vitro anticancer evaluation of iron oxide/chitosan nanocomposites. Inorg Nano Met Chem. 2017;47(3):405–411. doi: 10.1080/15533174.2016.1186064. [DOI] [Google Scholar]
- Behera SS, Patra JK, Pramanik K, Panda N, Thatoi H. Characterization and evaluation of antibacterial activities of chemically synthesized iron oxide nanoparticles. World J Nano Sci Eng. 2012;2(4):196–200. doi: 10.4236/wjnse.2012.24026. [DOI] [Google Scholar]
- Bharathi D, Ranjithkumar R, Vasantharaj S, Chandarshekar B, Bhuvaneshwari V. Synthesis and characterization of chitosan/iron oxide nanocomposite for biomedical applications. Int J Biol Macromol. 2019;132:880–887. doi: 10.1016/j.ijbiomac.2019.03.233. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MSH, Yusuf Miah M, Chandra Paul S, Das Aka T, Saha O, Rahaman MM, Sharif MJI, Habiba O, Ashaduzzaman M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon. 2020;6:e04603. doi: 10.1016/j.heliyon.2020.e04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campas-Baypoli ON, Sánchez-Machado DI, Bueno-Solano C, Ramírez-Wong B, López-Cervantes J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed Chromatogr. 2010;24(4):387–392. doi: 10.1002/bmc.1303. [DOI] [PubMed] [Google Scholar]
- Castelló J, Gallardo M, Busquets MA, Estelrich J. Chitosan (or alginate)-coated iron oxide nanoparticles: a comparative study. Colloids Surf A Physicochem Eng Aspects. 2015;468:151–158. doi: 10.1016/j.colsurfa.2014.12.031. [DOI] [Google Scholar]
- Cuong ND, Hoa TT, Khieu DQ, Lamd TD, Hoa ND, Hieu NV. Synthesis, characterization, and comparative gas-sensing properties of Fe2O3 prepared from Fe3O4 and Fe3O4-chitosan. J Alloys Compd. 2012;523:120–126. doi: 10.1016/j.jallcom.2012.01.117. [DOI] [Google Scholar]
- Deshmukh AR, Gupta A, Kim BS. Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. BioMed Res Int. 2019 doi: 10.1155/2019/1714358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavale RP, Dhavale RP, Sahoo SC, Kollu P, Jadhav SU, Patil PS, Dongale TD, Chougale AD, Patil PB. Chitosan coated magnetic nanoparticles as carriers of anticancer drug Telmisartan: pH-responsive controlled drug release and cytotoxicity studies. J Phys Chem Solids. 2021;148:109749. doi: 10.1016/j.jpcs.2020.109749. [DOI] [Google Scholar]
- El Zowalaty ME, Al Ali SHH, Husseiny MI, Geilich BM, Webster TJ, Hussein MZ. The ability of streptomycin-loaded chitosan-coated magnetic nanocomposites to possess antimicrobial and antituberculosis activities. Int J Nanomed. 2015;10(1):3269–3274. doi: 10.2147/IJN.S74469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Han E, He Y, Du C, Liu J, Yang X. Effect of different templating agents on cobalt ferrite (CoFe2O4) nanomaterials for high-performance supercapacitor. Ionics. 2020;26:3643–3654. doi: 10.1007/s11581-020-03482-z. [DOI] [Google Scholar]
- Gingasu D, Mindru I, Patron L, Ianculescu A, Vasile E, Marinescu G, Preda S, Diamandescu L, Oprea O, Popa M, Saviuc C, Chifiriuc MC. Synthesis and characterization of chitosan-coated cobalt ferrite nanoparticles and their antimicrobial activity. J Inorg Organomet Polym Mater. 2018;28:1932–1941. doi: 10.1007/s10904-018-0870-3. [DOI] [Google Scholar]
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signaling pathways. Biochem Soc Trans. 2001;29(2):345–350. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- Izadiyan Z, Shameli K, Miyake Hara MH, Mohamad SEB, Kalantari K, Taib SHM, Rasouli E. Cytotoxicity assay of plant-mediated synthesized iron oxide nanoparticles using Juglans regia green husk extract. Arab J Chem. 2018;13(1):2011–2023. doi: 10.1016/j.arabjc.2018.02.019. [DOI] [Google Scholar]
- Jagathesan G, Rajiv P. Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal Agric Biotechnol. 2018;13:90–94. doi: 10.1016/j.bcab.2017.11.014. [DOI] [Google Scholar]
- Jegadeesan GB, Srimathi K, Srinivas NS, Manishkanna S, Vignesh D. Green synthesis of iron oxide nanoparticles using Terminalia bellirica and Moringa oleifera fruit and leaf extracts: antioxidant, antibacterial and thermoacoustic properties. Biocatal Agric Biotechnol. 2019;21:101354. doi: 10.1016/j.bcab.2019.101354. [DOI] [Google Scholar]
- Kakavandi B, Bahari N, Kalantary RR, Fard ED. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason Sonochem. 2019;55:75–85. doi: 10.1016/j.ultsonch.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Kalaivani R, Maruthupandy M, Muneeswaran T, Hameedha Beevi A, Anand M, Ramakritinan CM, Kumaraguru AK. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front Lab Med. 2018;2(1):30–35. doi: 10.1016/j.flm.2018.04.002. [DOI] [Google Scholar]
- Kanagasubbulakshmi S, Kadirvelu K. Green synthesis of iron oxide nanoparticles using Lagenaria siceraria and evaluation of its antimicrobial activity. Def Life Sci J. 2017;2(4):422–427. doi: 10.14429/dlsj.2.12277. [DOI] [Google Scholar]
- Karthika V, AlSalhi MS, Devanesan S, Gopinath K, Arumugam A, Govindarajan M. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci Rep. 2020;10:18912. doi: 10.1038/s41598-020-76015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkouli KI, Mahani MT, Mosaddegh E. Synthesis and characterization of aminotetrazole-functionalized magnetic chitosan nanocomposite as a novel nanocarrier for targeted gene delivery. Mater Sci Eng C. 2018;89:166–174. doi: 10.1016/j.msec.2018.03.032. [DOI] [PubMed] [Google Scholar]
- Koli RR, Phadatare MR, Sinha BB, Sakate DM, Ghule AV, Ghodake GS, Deshpande NG, Fulari VJ. Gram bean extract-mediated synthesis of Fe3O4 nanoparticles for tuning the magneto-structural properties that influence the hyperthermia performance. J Taiwan Inst Chem Eng. 2019;95:357–368. doi: 10.1016/j.jtice.2018.07.039. [DOI] [Google Scholar]
- Konwar A, Gogoi A, Chowdhury D. Magnetic alginate–Fe3O4 hydrogel fiber capable of ciprofloxacin hydrochloride adsorption/separation in aqueous solution. RSC Adv. 2015;5(99):81573. doi: 10.1039/c5ra16404d. [DOI] [Google Scholar]
- Konwar A, Kalita S, Kotoky J, Chowdhury D. Chitosan−iron oxide coated graphene oxide nanocomposite hydrogel: a robust and soft antimicrobial biofilm. ACS Appl Mater Interfaces. 2016;8(32):20625–20634. doi: 10.1021/acsami.6b07510. [DOI] [PubMed] [Google Scholar]
- Kothavale VP, Chavan VD, Sahoo SC, Kollu P, Dongale TD, Patil PS, Patil PB. Removal of Cu(II) from aqueous solution using APTES-GA modified magnetic iron oxide nanoparticles: Kinetic and isotherm study. Mater Res Express. 2019;6(10):106103. doi: 10.1088/2053-1591/ab3590. [DOI] [Google Scholar]
- Kouhbanani MAJ, Beheshtkhoo N, Taghizadeh S, Mohammad Amani A, Alimardani V. One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv Nat Sci Nanosci Nanotechnol. 2019 doi: 10.1088/2043-6254/aafe74. [DOI] [Google Scholar]
- Kumar B, Smita K, Cumbal L, Debut A. Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication. J Saudi Chem Soc. 2014;18(4):364–369. doi: 10.1016/j.jscs.2014.01.003. [DOI] [Google Scholar]
- Lawrie G, Keen I, Drew B, Chandler-Temple A, Rintoul L, Fredericks P, Grøndahl L. Interactions between alginate and chitosan biopolymers characterizedusing FTIR and XPS. Biomacromol. 2007;8(8):2533–2541. doi: 10.1021/bm070014y. [DOI] [PubMed] [Google Scholar]
- Liao J, Liu P, Xie Y, Zhang Y. Metal oxide aerogels: Preparation and application for the uranium removal from aqueous solution. Sci Total Environ. 2021;768:144212. doi: 10.1016/j.scitotenv.2020.144212. [DOI] [PubMed] [Google Scholar]
- Lu Q, Zhang Y, Hu H, Wang W, Huang Z, Chen D, Yang M, Liang J. In situ synthesis of a stable Fe3O4@ cellulose nanocomposite for efficient catalytic degradation of methylene blue. Nanomaterials. 2019;9(2):275. doi: 10.3390/nano9020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Cheng Y, Wei X, Chen D, Zhao X, Jia P. Gold embedded chitosan nanoparticles with cell membrane mimetic polymer coating for pH-sensitive controlled drug release and cellular fluorescence imaging. J Biomater Appl. 2020 doi: 10.1177/0885328220952594. [DOI] [PubMed] [Google Scholar]
- Manikandan A, Sathiyabama M. Green synthesis of copper-chitosan nanoparticles and study of its antibacterial activity. J Nanomed Nanotechnol. 2015;6:1. doi: 10.4172/2157-7439.1000251. [DOI] [Google Scholar]
- Manikandan A, Sathiyabama M. Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int J Biol Macromol. 2016;84:58–61. doi: 10.1016/j.ijbiomac.2015.11.083. [DOI] [PubMed] [Google Scholar]
- Maria Dhivya S, Sathiya SM, Manivannan G, Jothi Rajan MA. A comparative study on the biopolymer functionalized iron oxide nanocomposite for antimicrobial activity. Mater Today Proc. 2016;3(10):3866–3871. doi: 10.1016/j.matpr.2016.11.042. [DOI] [Google Scholar]
- Mirza AU, Kareem A, Nami SAA, Shoeb Khan M, Rehman S, Bhat SA, Mohammad A, Nishat N. Biogenic synthesis of iron oxide nanoparticles using Agrewia optiva and Prunus persica phyto species: Characterization, antibacterial and antioxidant activity. J Photochem Photobiol B Biol. 2018;185:262–274. doi: 10.1016/j.jphotobiol.2018.06.0. [DOI] [PubMed] [Google Scholar]
- Mott D, Thuy NTP, Aoki Y, Maenosono S. Aqueous synthesis and characterization of Ag and Ag–Au nanoparticles: addressing challenges in size, monodispersed and structure. Philos Trans A Math Phys Eng Sci. 2010;368(1927):4275–4292. doi: 10.1098/rsta.2010.0120. [DOI] [PubMed] [Google Scholar]
- Muthukumar H, Manickam M. Amaranthus spinosus leaf extract mediated FeO nanoparticles: physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain Chem Eng. 2015;3(12):3149–3156. doi: 10.1021/acssuschemeng.5b00722. [DOI] [Google Scholar]
- Nehra P, Chauhan RP, Garg N, Verma K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br J Biomed Sci. 2018;75(1):13–18. doi: 10.1080/09674845.2017.1347362. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Nguyen NT, Nguyen VA. In situ synthesis and characterization of ZnO/chitosan nanocomposite as an adsorbent for removal of Congo Red from aqueous solution. Adv Polym Tech. 2020 doi: 10.1155/2020/3892694. [DOI] [Google Scholar]
- Noorisepehr M, Kakavandi B, Isaric AA, Ghanbari F, Dehghanifard E, Ghomie N, Kamran F. Sulfate radical-based oxidative degradation of acetaminophen over an efficient hybrid system: Peroxydisulfate decomposed by ferroferric oxide nanocatalyst anchored on activated carbon and UV light. Sep Purif Technol. 2020;250:116950. doi: 10.1016/j.seppur.2020.116950. [DOI] [Google Scholar]
- Noval VE, Carriazo JG. Fe3O4-TiO2 and Fe3O4-SiO2 core-shell powders synthesized from industrially processed magnetite (Fe3O4) microparticles. Mater Res. 2019;22:e20180660. doi: 10.1590/1980-5373-MR-2018-0660. [DOI] [Google Scholar]
- Osuntokun J, Onwudiwe DC, Ebenso EE. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem Lett Rev. 2019;12:444–457. doi: 10.1049/iet-nbt.2017.0277. [DOI] [Google Scholar]
- Ottonelli M, Zappia S, Demartini A, Alloisio M. Chitosan-stabilized noble metal nanoparticles: study of their shape evolution and post-functionalization properties. Nanomaterials. 2020;10:224. doi: 10.3390/nano10020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai S, Kini SM, Narasimhan M, Pugazhendhi A, Selvaraj R. Structural characterization and adsorptive ability of green synthesized Fe3O4 nanoparticles to remove acid blue 113 dye. Surf Interfaces. 2021;23:100947. doi: 10.1016/j.surfin.2021.100947. [DOI] [Google Scholar]
- Park HJ, Kim JK, Kim J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009;43(4):1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Pérez AG, Martínez EG, Águila CRD, Martínez DAG, Ruiz GG, Artalejo AG, Yeasde-Madeir H. Chitosan-coated magnetic iron oxide nanoparticles for DNA and rhEGF separation. Colloids Surf A. 2020;591:124500. doi: 10.1016/j.colsurfa.2020.124500. [DOI] [Google Scholar]
- Pham XN, Nguyen TP, Pham TN, Tran TTN, Tran TVT. Synthesis and characterization of chitosan coated magnetite nanoparticles and their application in curcumin drug delivery. Adv Nat Sci Nanosci. 2016;7:045010. doi: 10.1088/2043-6262/7/4/045010. [DOI] [Google Scholar]
- Podrepšek GH, Knez Z, Leitge M. Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials. 2020;10:1913. doi: 10.3390/nano10101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poka LP, KrishnaMohan G, Venkateswara Rao K, Shanker K. Biosynthesis, characterization and acute oral toxicity studies of synthesized iron oxide nanoparticles using ethanolic extract of Centella asiatica plant. Mater Lett. 2019;236:256–259. doi: 10.1016/j.matlet.2018.10.037. [DOI] [Google Scholar]
- Powell CD, Atkinson AJ, Ma Y, Marcos-hernandez M, Villagran D, Westerhoff P, Wong MS. Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J Nanopart Res. 2020;22:48. doi: 10.1007/s11051-020-4770-4. [DOI] [Google Scholar]
- Qiu X, Wang S, Miao S, Suo H, Xu H, Hu Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J Hazard Mater. 2021;401:123353. doi: 10.1016/j.jhazmat.2020.123353. [DOI] [PubMed] [Google Scholar]
- Qu JB, Shao HH, Jing GL, Huang F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: preparation, characterization and cytotoxicity studies. Colloids Surf B. 2013;102:37–44. doi: 10.1016/j.colsurfb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Rezaei SS, Dehghanifard E, Noorisepehr M, Ghadirinejad K, Kakavandi B, Esfahani AR. Efficient clean-up of waters contaminated with diazinon pesticide using photo-decomposition of peroxymonosulfate by ZnO decorated on a magnetic core/shell structure. J Environ Manage. 2019 doi: 10.1016/j.jenvman.2019.109472. [DOI] [PubMed] [Google Scholar]
- Ruíz-Baltazar A, Esparza R, Rosas G, Pérez R. Effect of the surfactant on the growth and oxidation of iron nanoparticles. J Nanomater. 2015 doi: 10.1155/2015/240948. [DOI] [Google Scholar]
- Ruíz-Baltazar AJ, Reyes-López SY, Mondragón-Sánchez ML, Robles-Cortés AI, Pérez R. Eco-friendly synthesis of Fe3O4 nanoparticles: Evaluation of their catalytic activity in methylene blue degradation by kinetic adsorption models. Results Phys. 2019;12:989–995. doi: 10.1016/j.rinp.2018.12.037. [DOI] [Google Scholar]
- Safari J, Javadian L. Chitosan decorated Fe3O4 nanoparticles as a magnetic catalyst in the synthesis of phenytoin derivatives. RSC Adv. 2014;4(90):48973–48979. doi: 10.1039/c4ra06618a. [DOI] [Google Scholar]
- Salah El-Din TA, Elzatahry AA, Aldhayan DM, Al-Enizi AM, Al-Deyab SS. Synthesis and characterization of magnetite zeolite nanocomposite. Int J Electrochem Sci. 2011;66:177–183. [Google Scholar]
- Samrot AV, Shobana N, Durga Sruthi P, Sai Sahithya C. Utilization of chitosan-coated superparamagnetic iron oxide nanoparticles for chromium removal. Appl Water Sci. 2018;8:192. doi: 10.1007/s13201-018-0841-4. [DOI] [Google Scholar]
- Shanmuganathan R, Immanuel Edison TNJ, Oscar FL, Kumar P, Shanmugam S, Pugazhendhi A. Chitosan nanopolymers: an overview of drug delivery against cancer. Int J Biol Macromol. 2019;130:727–736. doi: 10.1016/j.ijbiomac.2019.02.060. [DOI] [PubMed] [Google Scholar]
- Shrifian-Esfahni A, Salehi MT, Nasr-Esfahni M, Ekramian E. Chitosan-modified superparamagnetic iron oxide nanoparticles: design, fabrication, characterization and antibacterial activity. Chemist. 2015;69:26–32. [Google Scholar]
- Sivaraj R, Rahman PK, Rajiv P, Narendhran S, Venckatesh R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;129:255–258. doi: 10.1016/j.saa.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Sorasitthiyanukarn FN, Muangnoi C, Thaweesest W, Bhuket PRN, Jantaratana P, Rojsitthisak P, Rojsitthisak P. Polyethylene glycol-chitosan oligosaccharide-coated superparamagnetic iron oxide nanoparticles: a novel drug delivery system for curcumin diglutaric acid. Biomolecules. 2020;10:73. doi: 10.3390/biom10010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihasam S, Thyagarajan K, Korivi M, Lebaka VR, Mallem SPR. Phytogenic generation of NiO nanoparticles using stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules. 2020;10:89. doi: 10.3390/biom10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha V, Murugadoss G, Thangamuthu R. Structural and morphological tuning of Cu-based metal oxide nanoparticles by a facile chemical method and highly electrochemical sensing of sulphite. Sci Rep. 2021;11:3413. doi: 10.1038/s41598-021-82741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo H, Gao Z, Xu L, Xu C, Yu D, Xiang X, Huang H, Hu Y. Synthesis of functional ionic liquid modified magnetic chitosan nanoparticles for porcine pancreatic lipase immobilization. Mater Sci Eng C Mater Biol Appl. 2019;96:356–364. doi: 10.1016/j.msec.2018.11.041. [DOI] [PubMed] [Google Scholar]
- Thirumurugan A, Priyanka K, Priyanka A, Priyanka L, Raghavi J, Kumaresan K, Nithyapriya S. Synthesis and impregnation of Fe2O3 nanoparticles on cellulose paper and sodium alginate films for the preservation of fruit and vegetables. J Microbiol Biotech Food Sci. 2020;9(6):1166–1169. doi: 10.15414/jmbfs.2020.9.6.1166-1169. [DOI] [Google Scholar]
- Unsoy G, Yalcin S, Khodadust R, Gunduz G, Gunduz U. Synthesis optimization and characterization of chitosan coated iron oxide nanoparticles produced for biomedical applications. J Nanopart Res. 2012;14:964. doi: 10.1007/s11051-012-0964-8. [DOI] [Google Scholar]
- Varukattu NB, Vivek R, Rejeeth C, Thangam R, Ponraj T, Sharma A, Kannan S. Nanostructured pH-responsive biocompatible chitosan coated copper oxide nanoparticles: A polymeric smart intracellular delivery system for doxorubicin in breast cancer cells. Arab J Chem. 2020;13:2276–2286. doi: 10.1016/j.arabjc.2018.04.012. [DOI] [Google Scholar]
- Vasantharaj S, Sathiyavimal S, Senthilkumar P, Lewis Oscar F, Pugazhendhi A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J Photochem Photobiol B Biol. 2019;192:74–82. doi: 10.1016/j.jphotobiol.2018.12.025. [DOI] [PubMed] [Google Scholar]
- Xin H, Yang X, Liu X, Tang X, Weng L, Han Y. Biosynthesis of iron nanoparticles using tie guanyin tea extract for degradation of bromothymol blue. J Nanotechnol. 2016 doi: 10.1155/2016/4059591. [DOI] [Google Scholar]
- Yi Y, Tu G, Tsang PE, Xiao S, Fang Z. Green synthesis of iron-based nanoparticles from extracts of Nephrolepis auriculata and applications for Cr (VI) removal. Mater Lett. 2019;234:388–391. doi: 10.1016/j.matlet.2018.09.137. [DOI] [Google Scholar]
- Ying Y, Wu YB, Huang JY. Preparation and characterization of chitosan/poly(vinyl alcohol)/graphene oxide films and studies on their antibiofilm formation activity. J Biomed Mater Res A. 2020;108(10):2015–2022. doi: 10.1002/jbm.a.36961. [DOI] [PubMed] [Google Scholar]
- Yu R, Shi Y, Yang D, Liu Y, Qu J, Yu ZZ. Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-spectrum and rapid adsorption of water contaminants. ACS Appl Mater Interfaces. 2017;9(26):21809–21819. doi: 10.1021/acsami.7b04655. [DOI] [PubMed] [Google Scholar]
- Zamora-Moraa V, Gutiérrez MF, Román JS, Goya G, Hernández R, Mijangos C. Magnetic core–shell chitosan nanoparticles: rheological characterization and hyperthermia application. Carbohydr Polym. 2014;102:691–698. doi: 10.1016/j.carbpol.2013.10.101. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hu R, Sun D, Wu T, Li Y. Fabrication of chitosan/magnetite graphene oxide composites as a novel bioadsorbent for adsorption and detoxification of Cr (VI) from aqueous solution. Sci Rep. 2018;8:15397. doi: 10.1038/s41598-018-33925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.