Fig. 20. Ciliary muscle tension, intraocular pressure (IOP) and TM removal: Impact on outflow facility.
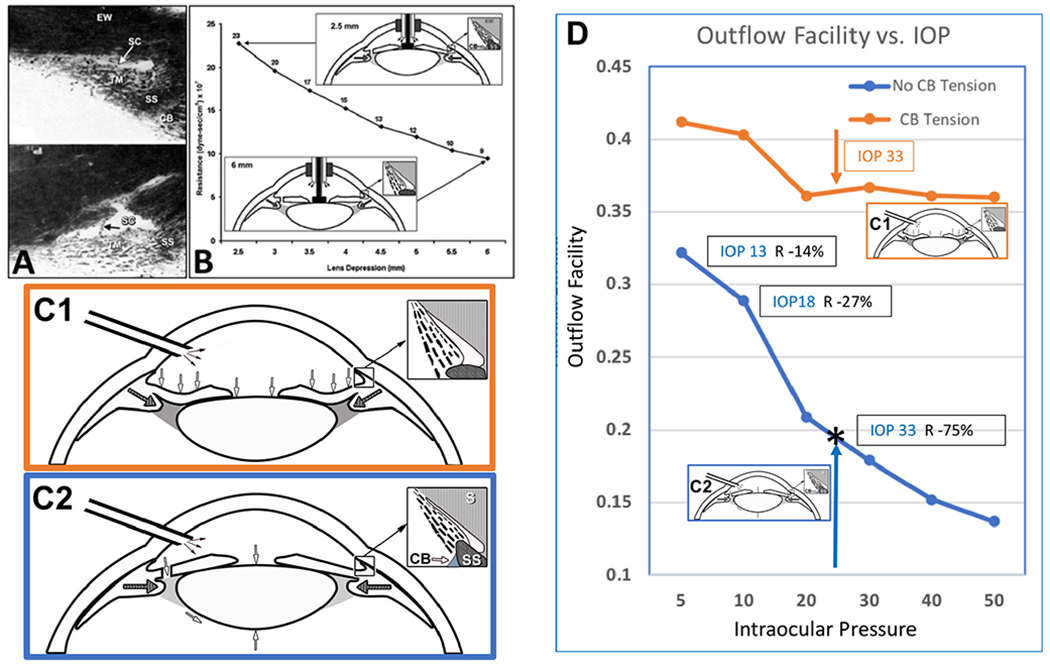
(A) Crystalline lens backward movement dilates Schlemm’s canal (SC) and reduces resistance. The upper panel is with no lens depression. The trabecular meshwork (TM) is in extensive apposition to SC external wall (EW), causing the closure of SC lumen (arrow). In the lower panel, with depression of the crystalline lens, the ciliary body (CB) and scleral spur (SS) rotate posteriorly, pulling the TM attachments away from SC external wall. The TM distends, and SC lumen is large. The black arrow demonstrates the SC inlet valve extending from the TM to a hinged flap at a collector channel entrance. (B) The corneal perfusion fitting contains a lens-depression device. The CB and SS move backward with lens depression resulting in the opening of SC. As the lens moves backward, causing CB tension, resistance falls by over 50%. (C1) No iridectomy. Anterior chamber perfusion forces the lens backward, resulting in a reverse pupillary block phenomenon. Backward movement increases zonular tension that transmits to the ciliary body, scleral spur (SS), and TM tendons. The tension and vector forces cause the scleral spur and TM to move posteriorly and inward. In (C2) with an iridectomy, pressure gradients equalize between the anterior and posterior chamber eliminating posterior lens movement, so no tension is exerted on the ciliary body. (D) Outflow facility experimentally controlled at a series of steady-state intraocular pressures. The blue curve is outflow facility with no ciliary body tension, as shown in the C2 protocol. The orange curve is outflow facility with ciliary body tension resulting from the protocol, as shown in Cl. Pressures in the abscissa need to be adjusted upward by 8 mm Hg to reflect transtrabecular in vivo pressure gradients because of the lack of episcleral venous pressure in the ex vivo setting. Ellingsen and Grant determined reduction of outflow resistance from TM removal at each of the pressures noted in the blue curve of C2. As indicated by the boxed data, an effective IOP of 13, 18, and 33 mm Hg, trabeculotomy reduces resistance (R) by 14%, 27%, and 75%, respectively. The upward-pointing blue arrow and asterisk indicate the conditions of Grant’s initial 1958 and 1963 studies. With simulated ciliary muscle tension as in condition C-2, the outflow facility is initially higher than under condition (C-1). The facility of outflow remains high despite increasing IOP. The authors conclude that it is an apposition of the TM to SC external wall that causes increased resistance with pressure. They also reach the conclusion that ciliary muscle tension prevents SC wall apposition and a decrease in outflow facility. (A) From Van Buskirk M, Anatomic correlates of changing outflow facility. Invest Ophthalmol Vis Sci 22, 625–632, 1982. (B) From Johnstone, Pump failure in glaucoma. Essentials in Ophthalmology: Glaucoma II. Springer, Heidelberg, 2006. C) From Ellingsen and Grant, IOP and aqueous outflow, Invest Ophthalmol 10: 430–437, 1971.
