Fig. 25. Tethering enables sensory signaling through cell deformation.
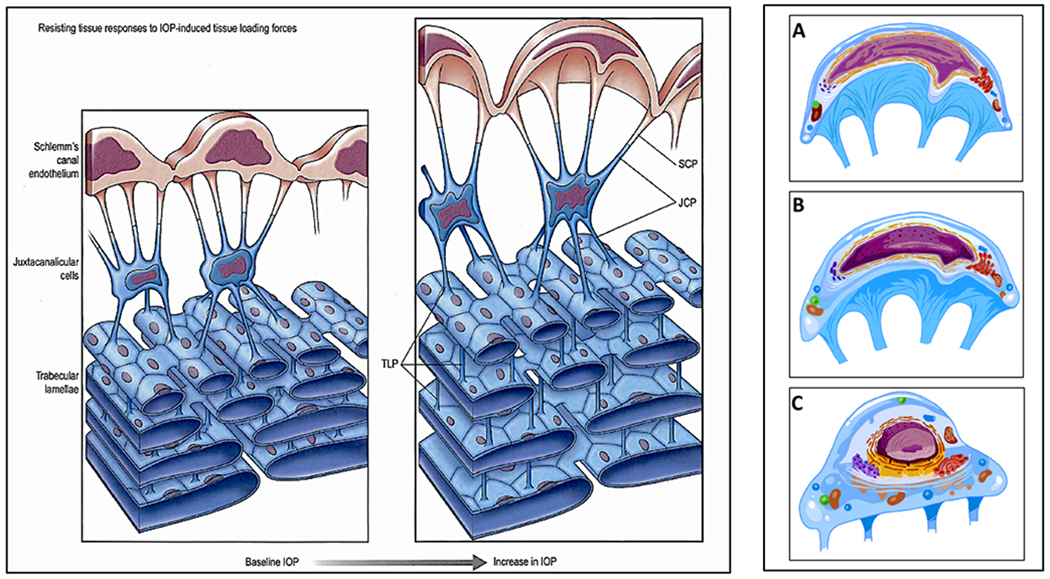
Cellular connections link the structural elements of the trabecular meshwork (TM). The cytoplasmic process tethering function permits Schlemm’s canal endothelial cell deformation. Left Panel Schlemm’s canal endothelial cells (SCE) have processes (SCP) that project into the juxtacanalicular space and attach to juxtacanalicular cell processes (JCP). JCP attach to trabecular lamellae endothelial cell processes (TIP), linking SCE to the trabecular lamellae. Trabecular lamellae endothelial cell cytoplasmic processes connect to adjacent trabecular lamellae cell processes. SC endothelial cell bodies, nuclei, and cytoplasmic processes undergo progressive deformation in response to progressive lOP-induced loading forces. The tethered trabecular lamellae limit SC inner wall endothelium distention by countering lOP-induced SCE loading forces. Spaces between the resisting trabecular tissues progressively increase as lOP increases. At physiologic pressures (baseline lOP), tensional integration is present throughout the trabecular tissues. IOP loading forces presented to SCE distribute throughout the TM lamellae as a result of the tethering cytoplasmic processes. The tensioned network allows finely graded responses to transient increases in lOP. Such force-dependent mechanotransduction mechanisms are like those elsewhere in the vasculature. Right Panel Detail of SC inner wall endothelium shown in panel one. The images in (A), (B), (C) depict alterations in cell surface membranes, organelles, nuclear envelope, nuclear intermediate filaments, and chromatin resulting from physiologic changes in IOP. The prestressed, tensionally-integrated chromatin permits instantaneous sensing of IOP changes at the genomic level. Pressure-dependent deformation of the cytoplasm, nucleus, and chromatin thus provides mechanotransduction signals to restore homeostatic setpoints (Section 5). The left panel is from “Aqueous Outflow Overview, Diagnosis and Therapy of the Glaucomas. Mosby, St. Louis, 22–46, 2009”. Right panel from Johnstone Glaucoma Lab, University of Washington.
