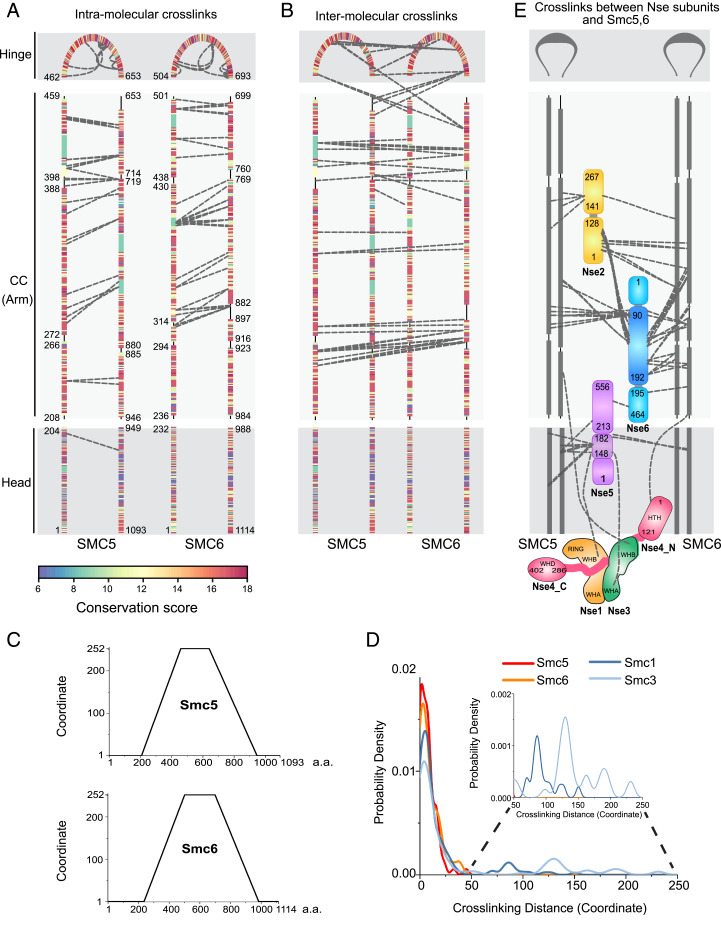
Fig. 2.
CL-MS analysis of Smc5/6 holo-complex. (A) Intra-Smc5 and intra-Smc6 cross-links. Three Smc5 and Smc6 regions, namely head, coiled-coil (CC, or arm), and hinge, are marked and residues bordering each region are labeled. Cross-links connecting the N- and C-terminal portions of each region are represented by dashed lines between the corresponding amino acid pairs. Cross-links connecting adjacent sequences listed in Dataset S1 are omitted to highlight the antiparallel nature of the coil-coiled pairing of Smc5 and Smc6. Coiled-coil regions are interrupted by noncoiled-coil segments and residues bordering each predicted coiled-coil segment are indicated. Amino acids are colored based on conservation scores as shown in SI Appendix, Fig. S1F and Dataset S3. (B) Intermolecular cross-links between Smc5 and Smc6. The graph is presented as in A. (C) Transformed amino acid coordinates for Smc5 and Smc6 (for details, see Materials and Methods). (D) Probability density of cross-linking distance based on transformed coordinates for Smc5 and Smc6 in the Smc5/6 complex, and for Smc1 and Smc3 in cohesin. CL-MS data for Smc1 and 3 are based on Bürmann et al. (8) (for details, see Materials and Methods and Dataset S2). (E) Intersubunits cross-links among Nse1-6 subunits, and between them and Smc5 and -6. Smc5 and Smc6 are presented similarly as in A and B. Domain structures of Nse subunits are not drawn to scale to highlight the cross-links. As shown previously, Nse1 contains WHA, WHB, and RING domains, while Nse3 contains WHA and WHB domains (23, 60), the Nse4 N-terminal region (Nse4_N) containing a helix-turn-helix (HTH) domain and its C-terminal region (Nse4_C) containing a WHD domain are connected by a largely unstructured region.