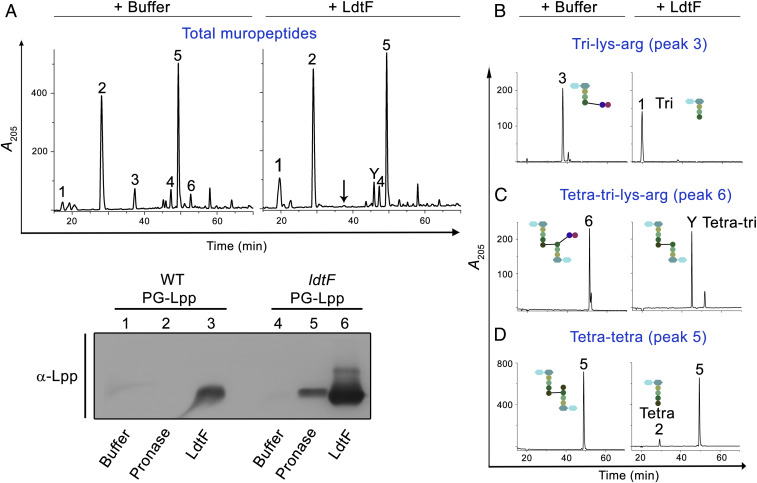
Fig. 4.
Endopeptidase activity of LdtF. Soluble muropeptides of WT PG sacculi (A), purified tri-lys-arg (B), purified tetra-tri-lys-arg (C), or purified tetra-tetra dimer (D) were incubated either with buffer or LdtF (4 μM) for 16 h and separated by RP-HPLC. LdtF cleaved peak 3 (tri-lys-arg) to yield tri (peak 1) and peak 6 (tetra-tri-lys-arg) to yield tetra-tri (peak Y). LdtF showed an extremely weak activity on tetra-tetra dimer (peak 5). (E) Cleavage of Lpp from the PG sacculi (containing bound Lpp) of WT or ldtF mutant was tested by incubating the PG sacculi either with buffer, pronase (0.2 mg/mL), or LdtF (4 µM) for 16 h at 30 °C. Pronase, a nonspecific protease, is used as a positive control.