Figure 3. Tpl2[D270A] mutation alters the protein composition of phagosomes.
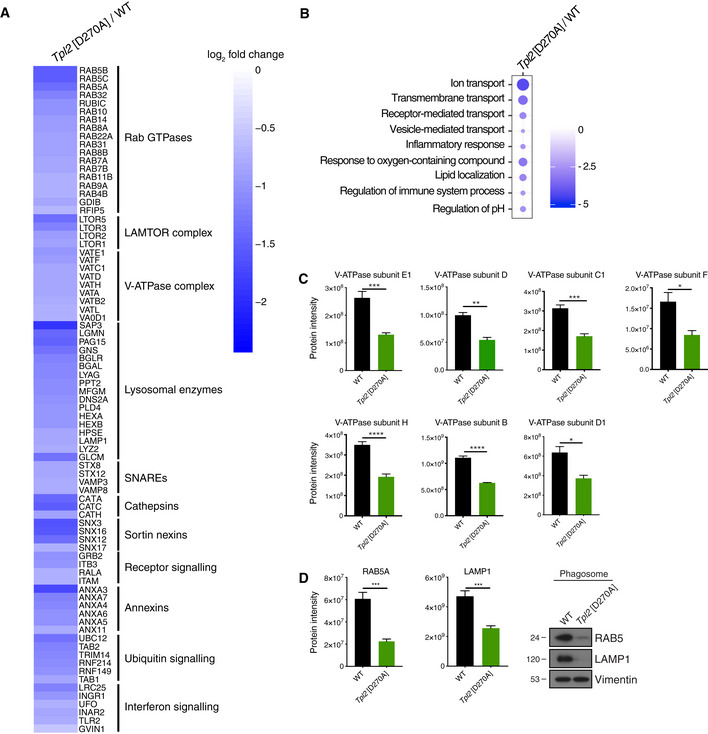
- Heatmap of selected proteins that were significantly downregulated in BMDMs from Tpl2[D270A] mice relative to WT (P < 0.05). Selected hits were grouped into clusters according to their molecular functions.
- Gene set enrichment analysis (GSEA) of significantly downregulated biological processes in phagosomal fractions. Changes of Tpl2[D270A] phagosomes relative to WT. Dot colour; enrichment score. Dot size; statistical significance.
- Protein intensities of V‐ATPase subunits from phagosomes purified from WT and Tpl2[D270A] BMDMs, (n = 3 biological replicates).
- Protein intensities of RAB5 and LAMP‐1 from phagosome proteome analysis of WT and Tpl2[D270A] BMDMs (n = 3 biological replicates) (left). Immunoblot of isolated phagosomes from WT and Tpl2[D270A] BMDMs probed for RAB5, LAMP‐1, and vimentin. Phagosomal fractions of two biological replicates were pooled. One representative experiment out of two shown (right) (n = 2).
Data information: Data were analysed by Student’s t‐test. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
