Abstract
Intervertebral disc degeneration (IDD) is widely recognized as the main cause of low back pain, which leads to disability in aging populations and induces great losses both socially and economically worldwide. Unfortunately, current treatments for IDD are aimed at relieving symptoms instead of preserving disc structure and function. Researchers are forged to find new promising biological therapeutics to stop, and even reverse, IVD degeneration. Recently, the injection of growth factors has been shown to be a promising biological therapy for IDD. A number of growth factors have been investigated to modulate the synthesis of the extracellular matrix (ECM) through a variety of pathogenetic biological mechanisms, including suppressing inflammatory process and down‐regulating degrading enzymes. However, growth factors, including Transforming Growth Factor‐β (TGF‐β), Fibroblast Growth Factor (FGF), and Insulin‐like Growth Factor‐1 (IGF‐1), may induce unwanted blood vessel in‐growth, which accelerates the process of IDD. On the contrary, studies have demonstrated that injection of GDF‐5 into the intervertebral disc of mice can effectively alleviate the degeneration of the intervertebral disc, which elicits their response via BMPRII and will not induce blood vessel in‐growth. This finding suggests that GDF‐5 is more suitable for use in IDD treatment compared with the three other growth factors. Substantial evidence has suggested that GDF‐5 may maintain the structure and function of the intervertebral disc (IVD). GDF‐5 plays an important role in IDD and is a very promising therapeutic agent for IDD. This review is focused on the mechanisms and functions of GDF‐5 in IDD.
Keywords: Biological therapy, Disc regeneration, Extracellular matrix metabolism, Growth and differentiation factor‐5, Intervertebral disc degeneration
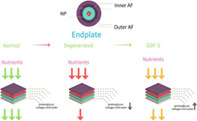
The nutritional supplies of inner AF and NP occur mainly through the endplate pathway. The normal endplate contains proteoglycan, collagen, and water, which will decrease substantially in the degenerated endplate. As the arrows show, all nutrients can pass through the normal cartilage endplate. When the endplate degenerates, passing nutrients are greatly reduced. After an injection of GDF‐5, there may be an increase in the delivery of nutrients.
Introduction
Low back pain (LBP) is a very common symptom worldwide. Most people have suffered from LBP at some time in their lives 1 . In America, according to statistics from 2008, about 25% of Americans have had back pain, and the related costs are estimated to be as high as US$85bn per year 2 . Therefore, LBP is becoming a great threat to human health. Studies have shown that intervertebral disc degeneration (IDD) is related to LBP, and there is a significant correlation between the degree of degenerated disc and the severity of lower back pain. IDD is characterized by apoptosis of function cells and breakdown of the extracellular matrix (ECM), especially in the nucleus pulposus (NP) 3 .
Intervertebral disc degeneration (IDD) is recognized as the main cause of LBP 4 . The intervertebral disc is composed of the nucleus pulposus, the annulus fibrosus, and the cartilage endplate, whose biochemical composition is mainly collagen and proteoglycan, providing the disc with its critical characteristics. IDD is defined as a progressive structural failure 5 , and is characterized by an imbalance between anabolism and catabolism of disc tissue. Its pathogenesis is very complicated and it involves a variety of pathological processes, including apoptosis, cell proliferation, degradation of the extracellular matrix, inflammation, and degeneration of cartilage endplates, which will lead to complex biochemical and molecular changes in the intervertebral disc, including the reduction of proteoglycan content, the conversion of collagen type II to collagen type I, and the decrease of NP cell density. These changes directly lead to the abnormity of IVD mechanical function and eventually cause structural damage, such as fibrous annulus rupture, NP cells protruding, etc. 6 . Unfortunately, current treatment methods for IDD are aimed at treating symptoms instead of regenerating disc structure and function 7 . Prevention or reversal of IDD is a potential treatment for LBP (Fig. 1).
Fig 1.
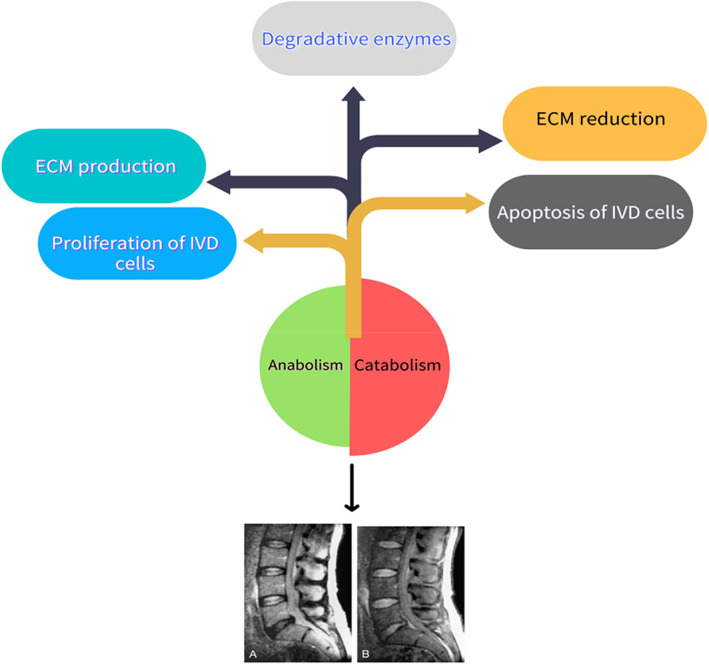
Imbalance between anabolism and catabolism in IVD cells leads and accelerates the process of IDD, which is shown by magnetic resonance imaging above.
GDF‐5 is known as cartilage‐derived morphogenetic protein‐1 8 and plays a significant role in various musculoskeletal processes 9 , such as endochondral ossification 10 , joint formation, and ligament maintenance. A number of studies suggested that GDF‐5 was closely related to IDD. An association between LDD and the SNP rs143383 (GDF‐5 gene) was identified, with the same risk allele as in knee and hip OA 11 . In degenerated intervertebral disc tissue, especially NP and AF, they found that the expression of GDF‐5 decreased. It is investigated that the extracellular matrix of discs from GDF‐5‐deficient mice is anomalous, and in vitro treatment with recombinant GDF‐5 can increase the expression of type II collagen and aggrecan genes 12 . In addition, the researchers also found that in the degenerated animal vertebral body model injected with GDF‐5 or transduced with GDF‐5 gene, the height of the intervertebral disc was obviously restored, and the synthesis of GAGS extra matrix increased after a few weeks. Various results have shown that GDF‐5 can repair degenerated intervertebral discs, slow and reverse IDD. It is imperative for people to clarify the mechanisms about GDF‐5 in the pathogenesis of IDD and find effective targeted drugs in treating IDD.
Search Strategy
(i) Searching platforms: Articles were hand‐searched from PubMed, a retrieval biomedical literature database. (ii) Databases: MEDLINE, OLDMEDLINE. (iii) Key words: GDF‐5, intervertebral disc degeneration. (iv) Boolean algorithm: GDF‐5 AND intervertebral disc degeneration. (v) Retrieving time: Issues of the selected journals published from 1999 to 2020 were hand‐searched by us. (vi) Inclusion and exclusion criteria: Articles were included if the study design was identified as an experimental or review article related to GDF‐5 and intervertebral disc degeneration. The search process was performed as presented in Fig. 2.
Fig 2.

Flow chart of the search for published reports showing the process of inclusion and exclusion.
GDF‐5 Affects ECM Metabolism in IVD
Matrix metalloproteinases (MMPs), members of the matrix‐degrading enzymes, were considered to play a pivotal role in disc tissue degradation and re‐absorption. A correlation was found between the increasing levels of matrix metalloproteinases 2 and 9, and the grade of degenerative disc disease 13 . By detecting the expression of MMPs, researchers determined that a leading function for MMPs in IVD degeneration, with the resulting loss of normal disc function, eventually led to low‐back pain 14 . In addition, studies indicated that miR‐155 is down‐regulated in degenerative nucleus pulposus, and more severe degeneration is correlated with higher matrix metallopeptidase 16 expression 15 . MMP‐16 may lead to the dehydration and degeneration of discs through degrading aggrecan and collagen type II. Related experiments have also proved that MMP‐3 has the same effect. Recently, mouse disc cells in vitro were treated with recombinant GDF‐5 protein, and therapy, with GDF‐5 protein and cDNA, was assessed by detecting cell proliferation, proteoglycan production, and extracellular matrix gene expression. Real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) demonstrated that the mGDF‐5 can suppress the expression of matrix metalloproteinase (MMP)‐3 16 . In other words, GDF‐5 can slow down the decomposition of ECM by inhibiting the expression of MMPs.
In the treatment of degenerative intervertebral discs, researchers used adenovirus‐mediated transduction of the GDF‐5 gene into the degenerated intervertebral disc tissue. The GDF‐5 gene was successfully expressed. The level of sGAG in the Ad‐GDF‐5 group was more than that in the BM or Ad‐GFP controls, and the level of sGAG gradually increased. NP cells treated with Ad‐GDF‐5 demonstrated robust staining for collagen II, whereas the cells infected by Ad‐GFP or those that were non‐infected showed weaker staining. Typically, the immunofluorescent staining intensity of aggrecan was higher toward the cytoplasm and lower in the nucleus regions of all cells. The results showed that Ad‐GDF‐5 is effective in promoting NP cell ECM secretion 17 (Table 1). In addition to promoting the synthesis of collagen and proteoglycan, Ad‐GDF‐5 can also up‐regulate the gene expression of type II collagen and aggrecan. However, by directly injecting GDF‐5 into the degenerated intervertebral disc, the researchers obtained similar experimental results. Researchers injected rhGDF‐5 loaded by PLAG into the degenerated intervertebral discs of mice and found that the height of the intervertebral discs was significantly restored, and the expression of type II collagen and proteoglycan genes were significantly up‐regulated 18 . Moreover, increases in glycosaminoglycan (GAG) and DNA content and an improvement in the differentiation index (the ratio of collagen type II to collagen type I) were also observed. (Table 2). Numerous experimental results show that GDF‐5 increases the synthesis of type II collagen and proteoglycan in order to alter the extracellular matrix metabolism of intervertebral disc cells into a synthetic state and enhances the anabolism of ECM.
TABLE 1.
GDF‐5 promotes the synthesis of the ECM
| MMPs | Collagen I | Collagen II | Aggrecan | sGAG | Hyp | |
|---|---|---|---|---|---|---|
| rhGDF‐5 | ↓ | ↓ | ↑↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
| Ad‐GDF‐5 | ↓ | ↓ | ↑↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
TABLE 2.
Gene expression patterns in human NP cells
| MMPs mRNA | CollagenI/β‐actin | CollagenIi/β‐actin | Aggrecan/β‐actin | sGAG/DNA | Hyp/DNA | |
|---|---|---|---|---|---|---|
| rhGDF‐5 | ↓ | ↓ | ↑↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
| Ad‐GDF‐5 | ↓ | ↓ | ↑↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
sGAG: The total proteoglycan level. Hyp: The total collagen content. Red arrows indicate important material changes and the number of arrows is positively correlated with the degree of change.
Vascular Endothelia Growth Factor (VEGF) is an important angiogenesis stimulating factor, which plays an important role in regulating tissue growth and regeneration. Therefore, the growth factor may be a pivotal factor in the development, maturation, and degeneration of human lumbar intervertebral discs in different stages and different states. People found the expression of VEGF in the intervertebral discs of the fetus and infants, indicating that VEGF may play a pivotal role in maintaining the presence of capillaries in the intervertebral disc during this period. Researchers did not find this factor in adult intervertebral discs. It is suggested that the disappearance of blood vessels caused by the disappearance of VEGF is an important cause of intervertebral disc degeneration. This would lead to a decrease in the nutrient content and oxygen tension in the intervertebral disc, and a significant down‐regulation in the level of cell metabolism, which leads to a decrease in collagen, proteoglycan, and water that maintain the biomechanical characteristics of the intervertebral disc, and ultimately causes the degeneration of the intervertebral disc 19 . Recently, studies have found that GDF‐5 can up‐regulate the expression of vascular endotheli, a growth factor that promotes angiogenesis of stromal cells and contributes to the synthesis of ECM.
GDF‐5 Influences The Nutritional Pathway
The nutritional metabolism of intervertebral disc is different from other tissues because of the absence of vascular. Annulus pathway and the endplate pathway are two main nutritional pathways in the intervertebral disc. The annulus fibrosus pathway is a secondary pathway, which mainly nourishes the outer tissues of the AF and has little nutritional effect on the inner fibrosus and nucleus pulposus. The cartilage endplate is the main way of nourishing the intervertebral disc 20 as this is how nutrients enter the nucleus pulposus and the internal fibrous annulus. The cartilage endplate is composed of a hyaline cartilage layer and a calcified cartilage layer 21 . In addition to delivering nutrients, the normal cartilage endplate can also relieve high‐load pressure, maintain the normal nutrient metabolism structure in the vertebral body, and ensure the inner fibrous ring and nucleus pulposus' nutritional supply.
The cartilage endplate is mainly composed of proteoglycan, collagen, and water, and the water content is slightly more than that of ordinary cartilage. The biochemical composition of the cartilage endplate is very important to maintain the integrity of the intervertebral disc. Its proteoglycan concentration has the ability to regulate material transport. The reduction of the proteoglycan of the cartilage endplate can cause the content of nucleus pulposus to decrease. One of the important mechanisms of IDD is cartilage endplate degeneration, which reduces nutrient supply 22 , leads to cell senescence and apoptosis, and increases the activity of the degradative enzyme MMP, thereby destroying the metabolic balance of ECM. Related experiments show that GDF‐5 can expand the AF fibrochondrocyte population to NP, and at the same time up‐regulate the expression of PG (proteoglycan), promote the synthesis of PG 23 , and inhibit the calcification of the cartilage endplate. By maintaining the nutritional pathways of the cartilage endplate, the process of IDD is inhibited (Fig. 3).
Fig 3.
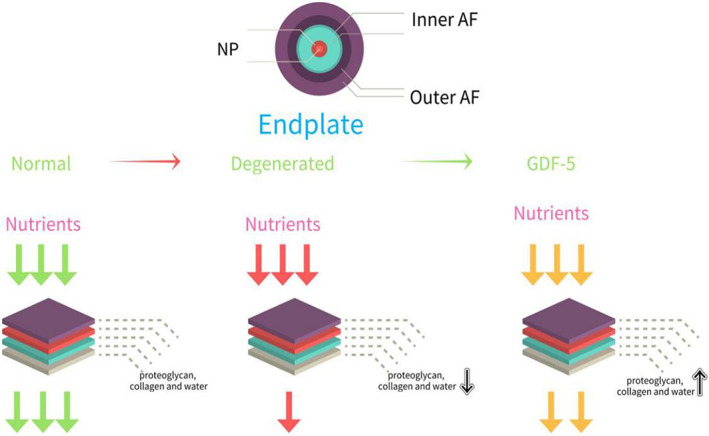
The nutritional supplies of inner AF and NP are mainly through endplate pathway. The normal endplate contains proteoglycan, collagen, and water, which will decrease substantially in the degenerated endplate. As the arrows show, all nutrients can pass through the normal cartilage endplate. When the endplate degenerates, passing nutrients are greatly reduced. After an injection of GDF‐5, there may be an increase in the delivery of nutrients.
GDF‐5 Inhibits Inflammatory Factors
The process of degeneration is accompanied by an increasing of inflammatory factors by IVD cells, such as TNF, IL‐1α, IL‐1β, IL‐6, and IL‐17, which has been implicated in disc herniation, nerve irritation, and in‐growth 24 . These cytokines trigger a range of pathogenic responses by the disc cells that can promote autophagy, senescence, and apoptosis 25 . They can not only up‐regulate a variety of catabolic mediators that include ADAMTS‐4/5, MMP‐1, ‐2, ‐3, ‐13, ‐14, and suppress the expression of important matrix genes, but they also induce changes in IVD cell phenotypes. This will induce structural changes and spinal instability, which are important causes of herniation, sciatica, and possibly stenosis 26 . More specifically, they can disrupt the balance of IVD tissue anabolism and catabolism, promote the degradation of extracellular matrix, and cause intervertebral disc herniation. Chemokines can promote the infiltration and activation of immune cells, thereby expanding the inflammatory cascade. In addition, neurogenic factors produced by the intervertebral disc and immune cells induce the expression of pain‐related cation channels of the dorsal root ganglion 27 , and the depolarization of these ion channels may promote discogenic and root‐induced pain 28 . So inflammatory factors are closely related to IDD, but the mechanism is very complicated, which promotes the process of IDD in a variety of ways (Fig. 4).
Fig 4.
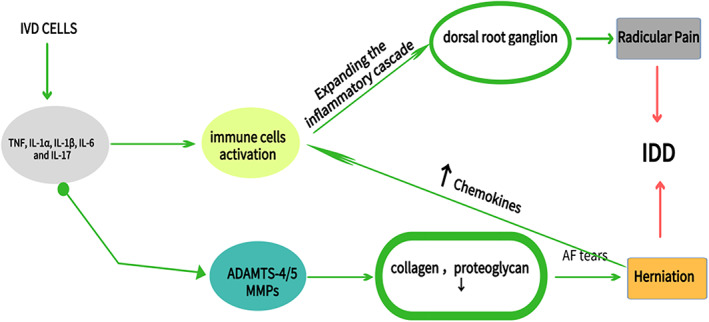
These pro‐inflammatory factors promote intervertebral disc degeneration. The imbalance between synthesis and catabolism further accelerates degeneration, compromises tissue integrity, and promotes pain generation.
Synthesis of cytokines, such as TNF‐α, IL‐1β, IL‐6, and IL‐8, are mediated by NF‐κB, which is one of the most important regulators of pro‐inflammatory gene expression 29 . Related studies have shown that GDF‐5 can inhibit the activation of the NF‐ κB signaling pathway, reduce the transcription of inflammatory factor genes, and down‐regulate the expression of inflammatory factors. Compared with the control group, protein expression levels of TNF‐α, IL‐1β, PGE2, and NO concentration were significantly repressed by GDF‐5 over expression (Table 3). Additionally, GDF‐5 over expression reduced LPS‐induced up‐regulation of TNF‐ α, IL‐1β, PGE2, iNOS, COX‐2, collagen‐II, aggrecan, IκBα, and p‐p65 expression levels in NP cells (Table 4). Taken together, these results demonstrated that GDF‐5 attenuated LPS‐induced IDD via inhibiting the production and release of inflammatory factors. However, the possible molecular mechanisms remain to be fully elucidated. As mentioned earlier, NP is mainly composed of collagen and proteoglycan, so the study also demonstrated that GDF‐5 prevented the loss of collagen II and aggrecan by inhibiting the NF‐ κB pathway. Collagen and proteoglycan destroyed by inflammatory factors had been significantly restored.
TABLE 3.
GDF‐5 down‐regulates the expression of inflammatory factors
| NF‐κB signaling pathway | TNF‐α | IL‐1β | PGE2 | NO | collagen | Aggrecan | |
|---|---|---|---|---|---|---|---|
| rhGDF‐5 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
| Ad‐GDF‐ 5 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
TABLE 4.
GDF‐5 inhibits relative mRNA level of inflammatory factors
| NF‐κB signaling pathway | TNF‐α/mRNA | IL‐1β/m RNA | COX2/mRN A | iNOS/mRN A | collagen/β‐actin | Aggrecan/ β‐actin | |
|---|---|---|---|---|---|---|---|
| rhGDF‐5 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
| Ad‐GDF‐ 5 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
The arrow indicates the change trend of the substance.
GDF‐5 Promotes The Proliferation Of IVD Cells
Several studies have suggested using anabolic growth factors to regenerate the normal matrix of the IVD and to restore disc height, thereby reversing degenerative disc disease. As a promising targeted drug for GDF‐5, we also need to find the receptor that binds to it. A related study investigated the expression and localization of four potentially beneficial growth factor receptors, suggesting that GDF‐5 mainly binds to BMPII receptors in the IVD cells. Not only can it participate in up‐regulating the proliferation of osteoblasts, periosteal cells, and fibroblasts, it can also promote the proliferation of NP cells, increase the number of functional cells in IVD, and inhibit the apoptosis of NP cells. However, a new treatment, a single injection of GDF‐5, was used to treat degenerative murine discs induced by static compression. After 4 weeks of treatment, it was observed that isogenic groups of fibrochondrocytes within the inner AF and NP increased substantially. The annulus fibrochondrocytes tended to expand into the hypocellular, degenerated NP cells, in responding to exogenous GDF‐5, which would express aggrecan and collagen type II genes and increase ECM synthesis of IVD 23 . And a single injection of recombinant human GDF‐5, in a rabbit model of disc degeneration, was also shown to improve the degenerated disc in both magnetic resonance imaging (MRI) findings and histological studies. MRI of NP of GDF‐5‐treated intervertebral discs showed stronger T2 signal intensity, indicating that the hydrophilicity of NP was maintained after GDF‐5 injection. In addition, the number of chondrocytes in NP and round chondrocytes in AF increased significantly 30 in the intervertebral disc. These results suggest that GDF‐5 can promote the expansion of annulus fibrochondrocytes and ECM synthesis, which increases cellularity, and the ECM content within the NP. As a mitogen for annulus fibrochondrocytes, GDF‐5 eventually restored the height and the biomechanical stability of the degenerative disc (Fig. 5).
Fig 5.
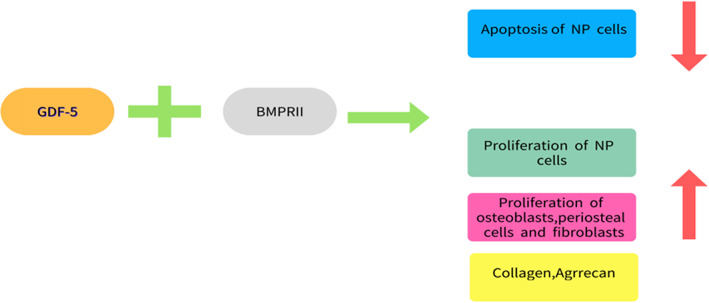
GDF‐5 mainly binds to BMPRII in the IVD cells, which can up‐regulate the proliferation of osteoblasts, periosteal cells, fibroblasts, and NP cells and inhibit the apoptosis of NP cells.
Discussion
Currently, it is concluded that GDF‐5 is mainly involved in three processes of IDD. Firstly, GDF‐5 is able to inhibit apoptosis of NP cells and promote the proliferation of IVD function cells. Some experiment results demonstrated that GDF‐5 overexpression prevents NF‐κB over‐activation in LPS‐stimulated nucleus pulposus cells. Previous studies have reported that the NF‐κB signaling pathway is aberrantly activated in IDD and is deeply involved in the pathogenesis of IDD. Therefore, we supposed that GDF‐5 confers protection against NPCs apoptosis through the regulation of NF‐κB signaling. But relative mechanisms have not been clarified. Secondly, GDF‐5 can significantly promote the synthesis of the main components of the extracellular matrix and rebalance the metabolism of that, especially the synthesis of type II collagen and aggrecan, which increase the production of sGAG. It is beneficial to the repair of degenerated intervertebral disc tissue and restore its physiological and mechanical functions. Both the height of the intervertebral discs and the effective components of the matrix have been significantly restored. Thirdly, GDF‐5 will down‐regulate the expression of pro‐inflammatory factors, reduce the inflammatory response, and thereby inhibit the inflammation‐induced breakdown of the ECM. It is particularly worth mentioning that GDF‐5 could inhibit the calcification of the endplates and the annulus fibrosus, through which the nutrient is delivered to the IVD, then prevent the degeneration of the intervertebral discs. Whether through in vitro or in vivo experiments, a large number of studies have shown that GDF‐5 has a significant effect on repairing degenerated intervertebral discs, which strengthens researchers' confidence in further research on GDF‐5. Research on the use of GDF‐5 in the treatment of IDD is also a hotspot of IDD. Experiments have shown that both adenovirus‐mediated GDF‐5 or rhGDF‐5 carried by PLGA can promote the synthesis of degenerative intervertebral disc matrix and restore the height of the intervertebral discs. Adenovirus successfully transduced the GDF‐5 gene into the degenerated intervertebral disc tissue. PLGA, as a carrier of rhGDF‐5, can release GDF‐5 for a long period of time, and sustainably maintain the concentration required for treatment. Moreover, as a targeted drug, GDF‐5 has a clear target of BMPII, which is mainly concentrated in the important tissues of intervertebral discs, such as NP and AF. More importantly, the receptor still evidences high activity in moderate to severe degeneration of intervertebral discs. Considered from many aspects, GDF‐5 as a targeted drug for the treatment of IDD has great research value and bright prospects. However, the current research also has a lot of limitations. The mechanisms of GDF‐5 in IDD are very complicated, and many signal pathways have not been clearly studied. Both in vivo and in vitro experiments are based on animal models, while human intervertebral disc experiments have only been carried out in vitro. Compared with the complex human internal environment, GDF‐5 may not ultimately work in the avascular intervertebral disc tissue as expected, and may even promote IDD. We do not know whether injection therapy is safe and reliable. Extensive clinical research is needed in the future (Fig. 6).
Fig 6.
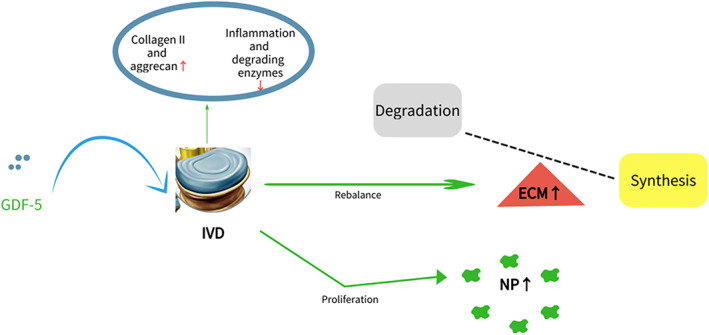
GDF‐5 prevents IVD against degeneration by rebalancing the metabolism of ECM and promoting the proliferation of NP cells.
Acknowledgments
Sheng Guo, Liqiang Cui, and Changming Xiao contributed equally to this work. I would like to express heartfelt gratitude to all the people who helped me in writing this paper.
Declaration: All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
Disclosure: The authors report no conflicts of interest.
Sheng Guo, Liqiang Cui and Changming Xiao are co‐first author and contributed to the work equally.
Contributor Information
Dingxuan Wang, Email: 635739608@qq.com.
Sen Li, Email: jht187@163.com.
References
- 1. Andersson GB. Epidemiological features of chronic low‐back pain. Lancet, 1999, 354: 581–585. 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA, 2008, 299: 656–664. 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 3. Vadalà G, Ambrosio L, Russo F, Papalia R, Denaro V. Interaction between Mesenchymal stem cell and intervertebral disc microenvironment: from cell therapy to tissue engineering. Stem Cells Int, 2019, 2019: 2376172. 10.1155/2019/2376172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari‐Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976)., 2000, 25: 487–492. 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 5. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976), 2006, 31: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 6. Li P, Zhang R, Zhou Q. Efficacy of platelet‐rich plasma in retarding intervertebral disc degeneration: a meta‐analysis of animal studies. Biomed Res Int, 2017, 2017: 7919201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng C, Liu H, Yang Y, Huang B, Zhou Y. Growth and differentiation factor‐5 contributes to the structural and functional maintenance of the intervertebral disc. Cell Physiol Biochem, 2015, 35: 1–16. 10.1159/000369670. [DOI] [PubMed] [Google Scholar]
- 8. Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta‐superfamily. Nature, 1994, 368: 639–643. 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 9. Francis‐West PH, Abdelfattah A, Chen P, et al. Mechanisms of GDF‐5 action during skeletal development. Development, 1999, 126: 1305–1315. [DOI] [PubMed] [Google Scholar]
- 10. Tsumaki N, Tanaka K, Arikawa‐Hirasawa E, et al. Role of CDMP‐1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol, 1999, 144: 161–173. 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams FM, Popham M, Hart DJ, et al. GDF5 single‐nucleotide polymorphism rs143383 is associated with lumbar disc degeneration in northern European women. Arthritis Rheum, 2011, 63: 708–712. 10.1002/art.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF‐5‐deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976), 2004, 29: 2229–2234. 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 13. Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine (Phila Pa 1976)., 1997, 22: 2877–2884. 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 14. Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J, 2002, 11: 308–320. 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang WL, Chen YF, Meng HZ, et al. Role of miR‐155 in the regulation of MMP‐16 expression in intervertebral disc degeneration. J Orthop Res, 2017, 35: 1323–1334. 10.1002/jor.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui M, Wan Y, Anderson DG, et al. Mouse growth and differentiation factor‐5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J, 2008, 8: 287–295. 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 17. Luo XW, Liu K, Chen Z, et al. Adenovirus‐mediated GDF‐5 promotes the extracellular matrix expression in degenerative nucleus pulposus cells. J Zhejiang Univ Sci B, 2016, 17: 30–42. 10.1631/jzus.B1500182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan J, Yang S, Sun H, et al. Effects of releasing recombinant human growth and differentiation factor‐5 from poly(lactic‐co‐glycolic acid) microspheres for repair of the rat degenerated intervertebral disc. J Biomater Appl, 2014, 29: 72–80. 10.1177/0885328213515034. [DOI] [PubMed] [Google Scholar]
- 19. Qing LH, Bao LF, Gu HY. The expression and its significance of vascular endothelial growth factor in lumbar inter vertebral discs. Chin J Spine Spinal Cord, 2002, 81: 39–41. [Google Scholar]
- 20. Ogata K, Whiteside LA. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine (Phila Pa 1976), 1981, 6: 211–216.7268543 [Google Scholar]
- 21. Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec, 1988, 220: 337–356. 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 22. Nerlich AG, Schleicher ED, Boos N. 1997 Volvo award winner in basic science studies. Immunohistologic markers for age‐related changes of human lumbar intervertebral discs. Spine (Phila Pa 1976)., 1997, 22: 2781–2795. 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 23. Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976)., 2004, 29: 156–163. 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 24. Hayashi S, Taira A, Inoue G, et al. TNF‐alpha in nucleus pulposus induces sensory nerve growth: a study of the mechanism of discogenic low back pain using TNF‐alpha‐deficient mice. Spine (Phila Pa 1976), 2008, 33: 1542–1546. 10.1097/BRS.0b013e318178e5ea. [DOI] [PubMed] [Google Scholar]
- 25. Shen C, Yan J, Jiang LS, Dai LY. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res Ther, 2011, 13: R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am, 2006, 88: 10–14. 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 27. Murata Y, Onda A, Rydevik B, Takahashi I, Takahashi K, Olmarker K. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor‐alpha to the dorsal root ganglion in rats. Spine (Phila Pa 1976)., 2006, 31: 530–535. 10.1097/01.brs.0000201260.10082.23. [DOI] [PubMed] [Google Scholar]
- 28. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol, 2014, 10: 44–56. 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tak PP, Firestein GS. NF‐kappaB: a key role in inflammatory diseases. J Clin Invest, 2001, 107: 7–11. 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor‐5 on the intervertebral disc–in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976)., 2006, 31: 2909–2917. 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]


