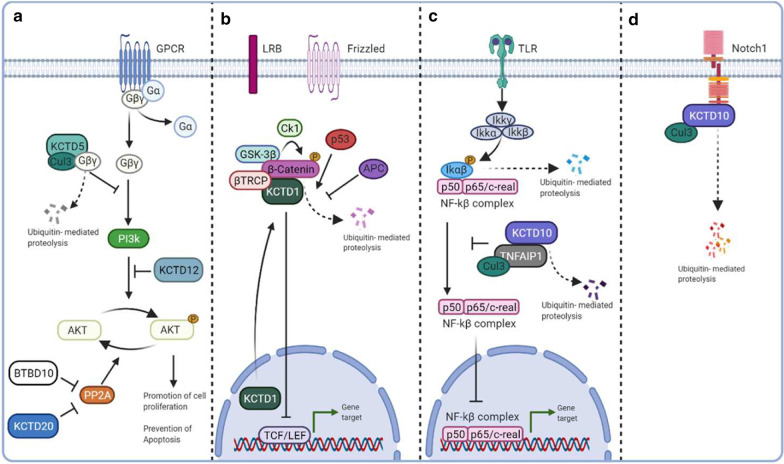
Fig. 2.
Schematic representation of the role of KCTDs in modulation of potentially tumorigenetic pathways. a: PI3K/AKT pathway. The activation of the GPCR receptor leads to the dissociation of the heterotrimer G into Gα and Gβγ. Subsequently, Gβγ activates PI3K that phosphorylates AKT. KCTD5 and KCTD12 negatively regulate the AKT pathway by triggering proteolysis of Gβγ heterodimer and inhibiting the phosphorylation of AKT, respectively. KCTD20 and BTBD10 suppress the PP2A-mediated dephosphorylation of AKT, acting as positive regulators of the pathway. b: WNT pathway. In the absence of Wnt ligand, β-catenin is degraded through the “destruction complex” (composed of CK1, axin, GSK-3β and APC), which phosphorylates β-catenin for ubiquitin-mediated proteasomal degradation. KCTD1 negatively regulates Wnt signalling by Enhancing β-catenin degradation. c: NF-κB pathway. Binding of inflammatory cytokines to TLR leads to the activation of the IKK complex, which phosphorylates IκBα. IκBα phosphorylation induces its ubiquitination and degradation by the proteasome, leading to nuclear translocation of the NF-κB transcriptional complex. TNFAIP1 interacts with KCTD10 inducing its degradation and inhibits the transcriptional activity of NF-κB. d: Notch pathway. KCT10 interacts with Cullin3 and the intracellular domain of Notch1, mediating Notch1 ubiquitination and proteolytic degradation