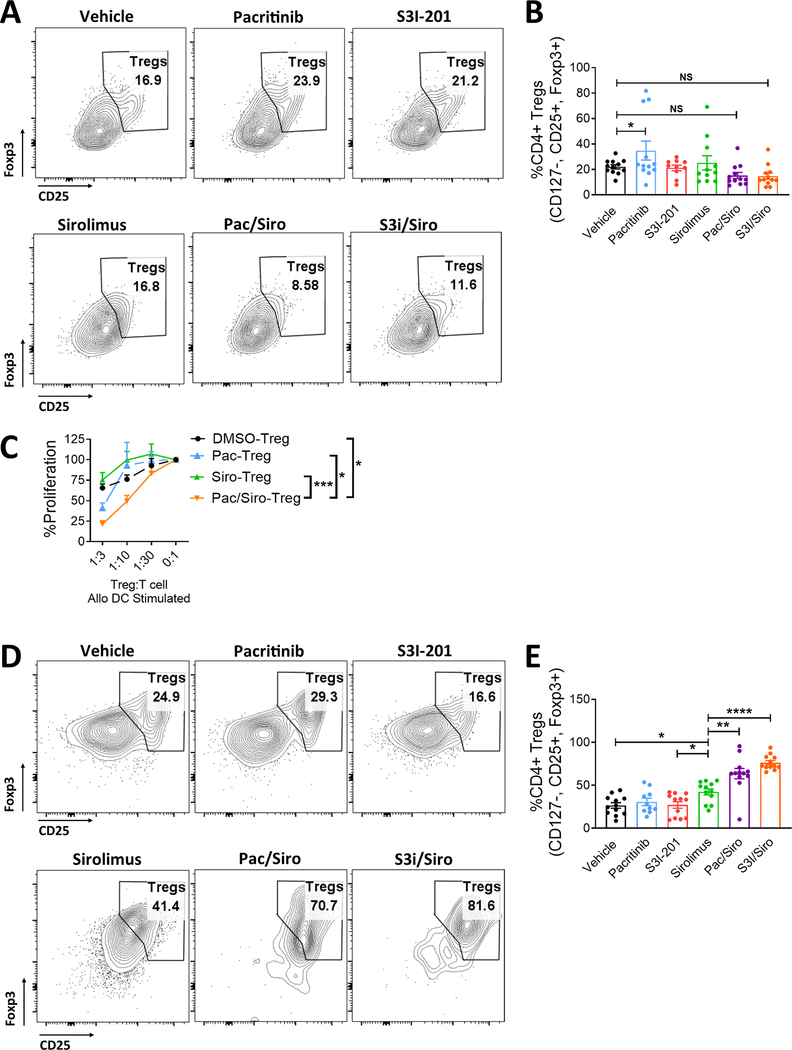
Figure 3. Concurrent JAK2 or STAT3/mTOR blockade maintains peripheral Tregs, but significantly improves Treg conversion from CD4+ Tconv.
Transplanted NSG mice treated with vehicle, pacritinib, S3I-201, sirolimus, pacritinib plus sirolimus, or S3I-201 plus sirolimus for 2 weeks were humanely euthanized at day +14. A) Representative contour plots and B) bar graph shows the frequency of human Tregs within the mouse spleens at day +14. Purified human peripheral Tregs were expanded with CD3/CD28 beads (Treg:bead ratio 1:1) for 5 days in the presence of vehicle, pacritinib 1.25μM, sirolimus 10ng/ml, or pacritinib plus sirolimus. Medium was supplemented with human recombinant IL-2 (20 IU/ml). C) The suppressive potency of vehicle-, pacritinib-, sirolimus-, or pacritinib plus sirolimus-treated Tregs was tested at different ratios of Treg to T cell responders in alloMLRs. No drugs were added to the suppression assay. Data are shown as mean ± SEM. N = 4 independent experiments. To investigate how dual JAK2 or STAT3 plus mTOR blockade impacts Treg induction in vivo, NSG mice were transplanted with Treg-depleted PBMCs. Mice received vehicle, pacritinib, S3I-201, sirolimus, pacritinib plus sirolimus, or S3I-201 plus sirolimus for 2 weeks as described. D) Representative contour plots and E) bar graph shows the frequency (mean ± SEM) of human Tregs recovered at day +14 for the mouse spleens. Pooled data from 3 independent experiments is shown, 12 mice per group. ANOVA (C, E). *P<0.05, **P=0.01–0.001, ***P=0.001–0.0001, ****P<0.00001.