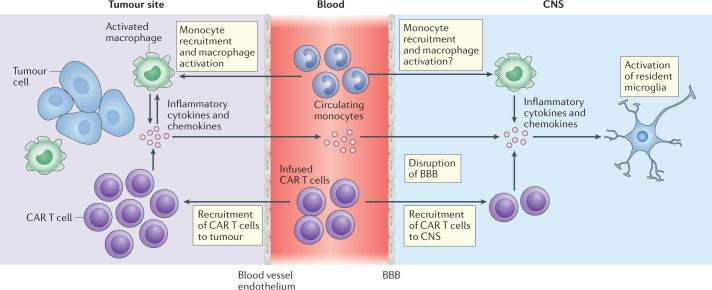
Fig. 4. Pathophysiology of ICANS.
Similar to cytokine release syndrome (CRS), the pathophysiology of immune effector cell-associated neurotoxicity syndrome (ICANS) seems to start with the production of pro-inflammatory cytokines by chimeric antigen receptor (CAR) T cells and the activation of bystander immune cells such as macrophages in the tumour microenvironment. Inflammatory cytokines and chemokines produced by CAR T cells and myeloid cells in the tumour microenvironment — such as IL-1β, IL-6, IL-10, the chemokines CXCL8 and CCL2, interferon-γ, granulocyte–macrophage colony-stimulating factor and tumour necrosis factor — diffuse into the bloodstream and, eventually, result in disruption of the blood–brain barrier (BBB), with accumulation of cytokines and CAR T cells in the central nervous system (CNS) together with activation of resident microglial cells.