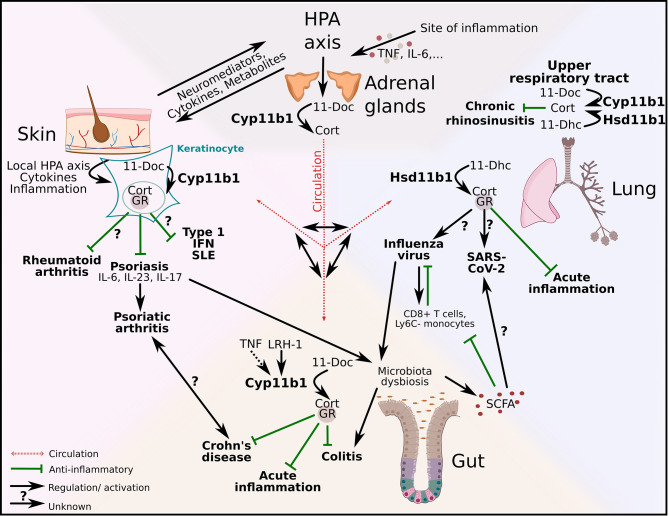
Figure 3.
Regulation of local type 1 and 3 immune responses and autoinflammatory diseases across and beyond epithelial barriers. Adrenal-derived GCs are regulated via the hypothalamus-pituitary-adrenal (HPA) axis. Inflammation triggers the HPA axis via cytokines (e.g. tumor necrosis factor (TNF), interleukin (IL)-6) and de novo synthesized GCs act systemically via the circulation. Additionally, HPA axis–skin communication occurs via neuromediators, cytokines and metabolites. GC synthesis in keratinocytes is regulated via a local HPA axis. Whereas the effect of local GCs on rheumatoid arthritis is currently unknown, GC-GR interactions have anti-inflammatory effects on psoriasis. In addition to that psoriasis alters the gut microbiota with substantial effects on intestinal homeostasis. The production of gut–derived GCs is regulated via liver receptor homolog-1 (LRH-1) and inducible via TNF. 11β-hydroxylase (Cyp11b1) catalyzes the conversion of 11-deoxycorticosterone (11-Doc) to corticosterone (Cort). Intestinal GCs exert anti-inflammatory effects on acute inflammation, experimental colitis and Crohn’s disease. Additionally, short chain fatty acids (SCFA) produced by the gut microbiota, have positive effects on influenza infection by modulating CD8+ T cell metabolism and increasing the number of Ly6C- monocytes. However, influenza virus has negative effects on the gut microbiota and can thereby limit the SCFA production. The influence of SCFA on other viral infections, like SARS-CoV-2, is currently not known. The synthesis of lung-derived GCs is catalyzed by 11β-hydroxysteroid dehydrogenase (Hsd11b1) and in the upper airways also by Cyp11b1. Local GCs inhibit acute lung inflammation. GC synthesis in the upper respiratory tract is upregulated in chronic rhinosinusitis patients and potentially exerts anti-inflammatory effects. The capacity of lung-derived GCs on skin-associated diseases and vice versa the effect of skin-derived GCs on lung disease in this context are unexplored.