Abstract
Vertebral malformations (VMs) are caused by alterations in somitogenesis and may occur in association with other congenital anomalies. The genetic etiology of most VMs remains unknown and their identification may facilitate the development of novel therapeutic and prevention strategies. Exome sequencing was performed on both the discovery cohort of nine unrelated probands from the USA with VMs and the replication cohort from China (Deciphering Disorders Involving Scoliosis & COmorbidities study). The discovery cohort was analyzed using the PhenoDB analysis tool. Heterozygous and homozygous, rare and functional variants were selected and evaluated for their ClinVar, HGMD, OMIM, GWAS, mouse model phenotypes, and other annotations to identify the best candidates. Genes with candidate variants in three or more probands were selected. The replication cohort was analyzed by another in-house developed pipeline. We identified rare heterozygous variants in KIAA1217 in four out of nine probands in the discovery cohort and in five out of 35 probands in the replication cohort. Collectively, we identified 11 KIAA1217 rare variants in 10 probands, three of which have not been described in gnomAD and one of which is a nonsense variant. We propose that genetic variations of KIAA1217 may contribute to the etiology of VMs.
Keywords: cervical vertebral fusion, KIAA1217, spine abnormalities, spine growth and development, vertebral malformation
1 |. INTRODUCTION
Vertebral malformations (VMs) have an approximate prevalence of 0.5–1 per 1,000 (Eckalbar, Fisher, Rawls, & Kusumi, 2012; Giampietro et al., 2009). They may occur in isolation or in association with other renal, cardiac, or spinal cord anomalies, or maybe part of an underlying syndromic diagnosis such as Klippel-Feil syndrome, Alagille syndrome, CHARGE, and others (Chen et al., 2016; Erol et al., 2004; Giampietro et al., 2009; Turnpenny et al., 2007). They may also be associated with the occurrence of congenital scoliosis or kyphosis (Erol et al., 2004; Giampietro et al., 2009).
VMs are caused by defects in the axial skeleton development. During embryogenesis, normal development and segmentation of the vertebrae involve the effective and highly orchestrated process of somitogenesis, which produces transient segments of tissue known as somites. The somites, comprising of paired metameric structures of paraxial mesoderm are located on both sides of the neural tube and form vertebrae, ribs, skeletal muscles, and dermis (Alazami et al., 2015; Erol et al., 2004; Hubaud & Pourquié, 2014; Mittmann et al., 2015; Sparrow, Chapman, Turnpenny, & Dunwoodie, 2007; Turnpenny et al., 2007). Disruption of somitogenesis results in various forms of VMs including defects of the formation such as wedge or hemivertebrae, defects of segmentation such as fused segments, and problems in midline fusion such as butterfly vertebrae (Eckalbar et al., 2012; Erol et al., 2004). VMs may be caused either by disruption of genes involved in the somitogenesis process, environmental insult during embryogenesis, or combination of both (Eckalbar et al., 2012; Erol et al., 2004).
Beauregard-Lacroix et al. (2017) identified a chromosomal anomaly by array comparative genomic hybridization in 31.3% of 32 patients with VMs. The chromosomal anomalies included trisomy 8 mosaicism, 22q11.2 deletion, 16p11.2 microdeletion, balanced and unbalanced translocations, pericentric inversion of chromosome 8, and inv(8)(q22.2q23.3) (Beauregard-Lacroix et al., 2017). More recently, genes such as CDH7 (Patten et al., 2012), GDF3 (Ye et al., 2010), GDF6 (Tassabehji et al., 2008), MEOX1 (Bayrakli et al., 2013), RIPPLY2 (Karaca et al., 2015) PAX1 (McGaughran, Oates, Donnai, Read, & Tassabehji, 2003), MYO18B (Alazami et al., 2015) TBX6 (Chen et al., 2020; Liu et al., 2019; Wu et al., 2015), SMARCC1 (Al Mutairi, Alzahrani, Ababneh, Kashgari, & Alkuraya, 2018), and T(brachyury) (Ghebranious et al., 2008) have been described as causing known autosomal dominant or autosomal recessive syndromes associated with different forms of VMs. Pathogenic variants in genes involved in the NOTCH signaling pathway (Dll3, Lfng, Mesp2, Hes7, and Tbx6) are known to produce a variety of somite or vertebral and rib abnormalities in mice (Erol et al., 2004; Mittmann et al., 2015; Sparrow et al., 2007; Turnpenny et al., 2007). Nevertheless, the genetic etiology for the majority of VMs is still unknown.
VMs cause significant functional distress such as chronic pain (caused by degenerative disc disease and myelopathy) and cosmetic disfigurement (Giampietro et al., 2009). Understanding their genetic etiology may aid in identifying novel treatment and prevention strategies.
In this study, we describe the identification of KIAA1217 as a novel candidate gene implicated in causing VMs with or without heart and central nervous system anomalies.
2 |. MATERIAL AND METHODS
2.1 |. Human subjects
2.1.1 |. USA centers
Probands with VM were invited to participate in an IRB approved study to identify novel genes associated with VM. Recruitment sites in the USA included the Hospital for Special Surgery, Marshfield Clinic, and the University of Wisconsin, Madison. From Marshfield Clinic, 95 probands were recruited. From the University of Wisconsin, Madison, 78 probands and five stillbirths were recruited. From the Hospital for Special Surgery, 13 probands were recruited. Spine films were reviewed by a study team (P.F.G., C.R., K.N., M.H., and B.N.) for inclusion. The medical record and corresponding X-rays of each study participant were reviewed to identify and confirm the specific VM phenotype, associated birth defects in the spinal cord, heart, kidneys, brain, and other skeletal abnormalities (Figure 1 and Table 1). X-rays were reviewed by the attending orthopedic surgeon involved with the case and study team in order to confirm a proband’s VM phenotype.
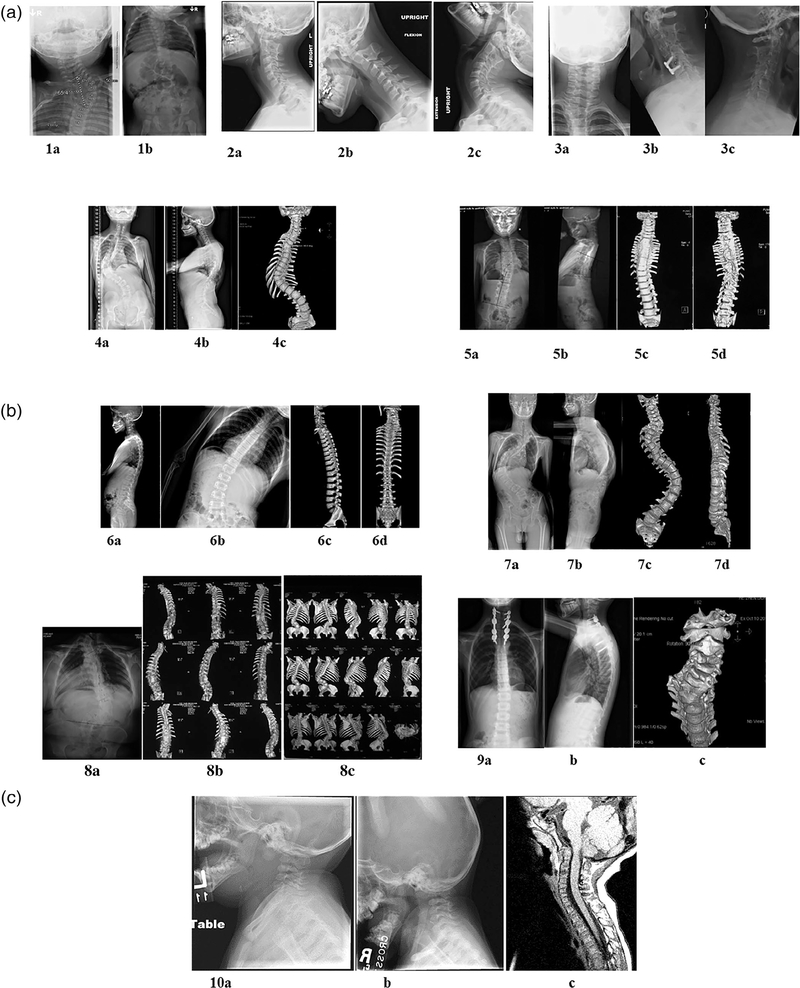
FIGURE 1.
Radiographic illustration of vertebral malformations seen in all probands. (a) Proband 1: Plain X-rays showing fused cervical and thoracic vertebrae in a 9-month-old male (1a, 1b). Proband 1 also have ASD. Proband 2, lateral neck films (2a) with flexion (2b) extension (2c) views showing fused cervical vertebrae in a 13-year-old male. Proband 2 also have macrocephaly, hydrocephalus, developmental delay, and intellectual impairment. Proband 3, 42-year-old male with dextrocardia, VSD. AP (3a), lateral (3b), and flexion (3c) X-rays showing fused cervical vertebrae. Proband 4, 12-year-old female with vertebrae deformation of C6, T3, T10, and segmentation defects of C3-C4, C6-C7, T3-T4 as shown in AP (4a) and lateral spine (4b) X-rays and AP 3D spine imaging (4c). Proband 5, AP (5a) and lateral spine (5b) X-ray and AP (5c) and PA (5d) 3D imaging of the spine showing extensive segmentation defects from C7-T9, thoracic spondylosis, tethered cord in 8-year-old male. (b) Proband 6, 10 year old male, AP (6a) and lateral (6b) X-ray and 3D spine imaging (6c and 6d) showing segmentation defects of C2/C3, non-segmented hemivertebrae of T4, and non-segmented wedged vertebrae of T12. Proband 7, 16-year-old male with segmentation defect of C6, non-segmented hemivertebrae of C7, and wedged vertebrae of T6/T7 as shown in AP (7a) and lateral (7b) X-ray and 3D imaging (7c and 7d). Leg-length discrepancy can be noted on the AP X-ray. Proband 8, 42-year-old female with C5-T5 segmentation defect with a short neck and non-segmented hemivertebrae of L1 as demonstrated by AP (8a) X-ray and 3D imaging (8b and 8c) of the spine. (i) Proband 9, spine C2-C7 segmentation defect in a 9-year-old male. AP (9a) and lateral spine X-ray (9b) and 3D imaging (9c) showing C-spine fusion. (c) Proband 10, lateral neck X-ray (10a and 10b) and MRI (10c) demonstrating incomplete segmentation of C1 fusion to Clivus, C3-C4, and hypoplastic occipital condyles
TABLE 1.
Clinical features of each proband outlined by major systems involved
| Clinical features |
|||||||
|---|---|---|---|---|---|---|---|
| Subject | Vertebral malformations | Cardiac | CNS | KIAA1217 variant (transcript NM_019590, build 37/hg19) | HVAR | CADD | gnomAD Frequency |
| Proband 1 | C+, T ± Fusion | ASD | Exon3: c.G433A:p.Ala145Thr | 0 | – | 1.789e-4 | |
| Proband 2 | C Fusion | Hydrocephalus Macrocephaly | Exon19: c.A4219T:p.Lys1407X | 0.73 | 8.03 | 0 | |
| Proband 3 | C Fusion | Dextrocardia and VSD | Tethered cord | Exon6: c.C1039T:p.Pro347Ser | 0.81 | 4.91 | 8.127e-6 |
| Proband 4 | C Fusion | Exon13: c:G2503T:pAla835Ser | 0.997 | 14.39 | 0 | ||
| T VM¶ | Exon13: c:C2660T:pAla887Val | 0.013 | 0.01 | 4.587e-4 | |||
| Proband 5 | C, T Fusion | Tethered cord | Exon19: c.5039 T > C:p.Ile1680Tyr | 0.964 | 15.64 | 2.032e-5 | |
| Proband 6 | C Fusion | Myocarditis | Exon17: c.5800G > T:p.Ala1934Ser | 0.01 | 5.177 | 1.931e-4 | |
| T VM | |||||||
| Proband 7 | C Fusion T VM | Exon14: c.3131C > T:p.Ser1044Leu | 0.67 | 28.7 | 2.924e-5 | ||
| Proband 8 | C Fusion T, L§ VM | Exon19: c.3877A > G:p.Ile1293Val | 0 | 0 | 1.234e-4 | ||
| Proband 9 | C Fusion | Exon7: c.901G > A:p.Gly301Arg | 12.9 | 12.9 | 2.853e-5 | ||
| Proband 10 | C Fusion incomplete segmentation | Small ASD VSD | Cerebellar tonsillar prolapse into spinal canal Basilar invagination Sprengel deformity |
Exon2: c.A185G:p.Asn62Ser | 0.337 | 18.12 | 3.934e-4 |
Note: +Cervical, ± Thoracic
Lumbar, and
Vertebral; Probands 1–3, 10 USA; Probands 4–9 Peking Union Medical College Hospital.
Details of each variant identified and their gnomAD frequency. Proband 2 also has an Xq21.1 deletion, which contains exons 1–29 of the BRWD3 gene. LOF variants in BRWD3 are associated with X-linked non-syndromic intellectual disability and macrocephaly. However, this does not explain the vertebral phenotype observed in this individual. +Cervical, ± Thoracic, § Lumbar, and ¶ Vertebral malformation.
DNA was extracted from blood or saliva specimens. Exome sequencing and analysis were subsequently performed on a subset of eight probands with cervical VM at the Baylor-Hopkins Center for Mendelian Genomics (BHCMG). DNA from Proband 10, also recruited from Marshfield Clinic, was sequenced at Victor Chang Cardiac Research Institute.
2.1.2 |. Peking Union Medical College Hospital, China
Thirty-five probands with cervical VMs delineated on X-ray/MRI were recruited between October 2010 and November 2017 as part of the Deciphering Disorders Involving Scoliosis and COmorbidities (DISCO) study (http://www.discostudy.org/).
The study was approved by the Human Subjects Ethics Committee of Peking Union Medical College Hospital and informed consent was obtained from probands or their parents. Evaluation of the VMs was performed using X-ray and CT, which were reviewed by two Orthopedic surgeons (N.W. and J.Z.) in a blinded fashion. Birth defects in other organ systems were also noted (Figure 1; Table 1).
DNA was extracted from peripheral blood according to a standardized protocol.
2.2 |. Exome sequencing
For samples sequenced at the BHCMG, DNA samples were processed with an Illumina HumanCoreExome-24v1–1 array to confirm gender, identify unexpected duplicates and relatedness, confirm study duplicates and relatedness, provide sample performance information and sample identity confirmation against the sequencing data. The Agilent SureSelect HumanAllExonV4_51MbKit_S03723314 was used for exome capture. Libraries were sequenced on the HiSeq2500 platform with onboard clustering using 125 bp paired-end runs and sequencing chemistry kits HiSeq PE Cluster Kit v4 and HiSeq SBS Kit v4. Intensity analysis and base calling were performed through the Illumina Real Time Analysis (RTA) software (version 1.17.20).
Variant filtering was done using the Variant Quality Score Recalibration method (Depristo et al., 2011; van der Auwera et al., 2014). For SNVs, the annotations of MQRankSum, QD, FS, SOR, and ReadPosRankSum were used in the adaptive error model. Summary statistics on the multi-sample were calculated for each variant (PASS and FAIL) including counts and frequencies of alleles and genotypes, missing rates, overall quality scores, and mean depth. In addition, separate summary statistics files for just Coriell control samples (HapMap and 1,000 Genomes subjects) and just BHCMG subjects for only PASS variants were generated.
DNA from patient 10 was sequenced at Victor Chang Cardiac Research Institute where exome sequencing, bioinformatic processing, and variant prioritization were carried out as described previously (Szot et al., 2018).
For samples analyzed in China, Illumina paired-end libraries were prepared from DNA samples and subjected to exome capture using the VCRome SeqCap EZ Chice HGSC 96 Reactions capture reagent (Roche), followed by sequencing on an Illumina HiSeq 4000 platform. The variant calling and annotation were performed by the in-house developed PUMP (Peking Union Medical College Hospital Pipeline) (Wang et al., 2018). In brief, single nucleotide variants and internal duplications and/or deletions (indels) were called using the HaplotypeCaller of the Genome Analysis Toolkit, version 3.4.0. Annotation for the de novo, compound heterozygotes, and homozygous inherited variants were calculated with Gemini (version 0.19.1) for in silico subtraction of parental variants from the proband’s variants, with accounting for read number information extracted from BAM files.
2.3 |. Variant filtering strategy
For samples sequenced at the BHCMG, we used the PhenoDB analysis tool (Sobreira, Boehm, Valle, & Hamosh, 2015) to select the heterozygous and homozygous, rare (MAF < 1%) and functional (missense, nonsense, stop loss, splicing, and indels) variants in each proband. Each of the variants and genes selected were evaluated for their ClinVar, HGMD, OMIM, GWAS, and mouse phenotype annotations among others. Next, we also selected the genes with heterozygous and/or homozygous candidate variants in three or more individuals. Upon identification of KIAA1217 as a candidate gene, we looked for individuals with rare functional variants in KIAA1217 among 35 individuals from the DISCO study.
2.4 |. Enrichment analysis
An enrichment analysis was performed on KIAA1217 using 994 unrelated individuals from the BHCMG project without known skeletal anomalies as controls and 44 probands with VMs. A contingency table containing the number of individuals presenting rare (MAF < 0.5%) missense, nonsense, stop loss, and/or splicing variants was built. Fisher’s exact test was determined and p < .05 was considered statistically significant.
3 |. RESULTS
3.1 |. Phenotypic description of probands
Unrelated probands included in this report showed variable forms of cervical VMs. Radiographic illustrations of various forms of VMs seen in all probands included in this report are shown in Figure 1. In addition to VMs, the probands had additional anomalies affecting their central nervous system (tethered cord, Chiari malformation; agenesis/absent corpus callosum; hydrocephalus, developmental delay), cardiac system (dextrocardia, VSD, ASD), gastrointestinal system (inguinal hernia, pyloric stenosis), genitourinary system (horseshoe kidney), and skeletal system (bifid ribs). A detailed phenotypic description of each proband is outlined in Table 1.
3.2 |. Candidate gene identification and variants details
The variant analysis for each proband ensured the investigation of variants in genes that are known to be associated with VMs including DLL3, LFNG, MESP2, HES7, TBX6, CDH7, GDF3, GDF6, MEOX1, RIPPLY2, PAX1, and MYO18B. We did not identify any candidate variants in these genes in any of the eight probands sequenced as part of the BHCMG. Next, we identified genes with candidate variants in three or more of the eight BHCMG probands. Among these genes, we identified rare heterozygous variants in KIAA1217 (also known as Sickle Tail; SKT) in three unrelated probands (Probands 1, 2, and 3; Table 1). Among the DISCO study, we identified an individual (Proband 4) with rare compound heterozygous variants in KIAA1217 (c.G2503T, p. Ala835Ser and c.C2660T, p.Ala887Val) and five individuals with rare heterozygous variants in KIAA1217 (Table 1). In an additional cohort from Victor Chang Cardiac Research Institute, we identified an individual (Proband 10) with a rare KIAA1217 variant (c.A185G, p. Asn62Ser). Details of KIAA1217 variants identified in each proband, including their gnomAD frequency, are outlined in Table 1. All the variants identified were missense with the exception of one, p.Lys1407*, variant identified in Proband 2.
Furthermore, our enrichment analysis identified 43 rare missense variants in 53 individuals among the 994 control individuals (Figure 2) with a statistically significant difference in the burden for KIAA1217 variants when comparing the patient group (44 probands) to the control group (p = .00016, Fisher’s exact test).
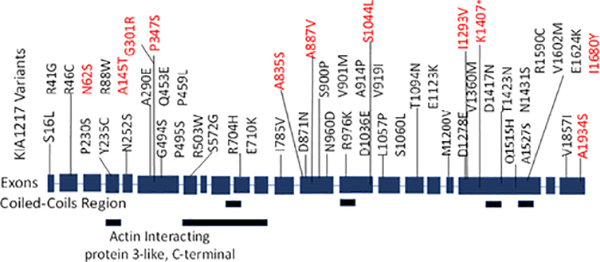
FIGURE 2.
Schematic representation of variants identified in KIAA1217. Forty-three variants (black) were identified in 994 control subjects. Eleven variants (red) were identified in our cohort. Two variants (p.Ala145Thr and p.Asn62Ser) were seen in a patient and a control subject. All variants were missense except p.Lys1407*
4 |. DISCUSSION
The recent advancement in molecular biology techniques has significantly enhanced our understanding of the genetic etiologies of VMs. To highlight the genetic and phenotypic heterogeneity associated with VMs, we conducted an OMIM search using the terms “vertebrae” and “malformation.” A total of 58 conditions were identified and summarized according to the pathways possibly affected in Table S1. The search identified multiple developmental pathways including Notch, Wnt, TGFβ among others, and genes closely involved with somite segmentation (Sparrow et al., 2007). Another 50–100 genes have been flagged as potentially expressed in a cyclic fashion in the presomitic mesoderm in mice representing a rich source for future investigations (Dequéant et al., 2006).
In our study, we identified rare heterozygous functional variants in KIAA1217 in 10 individuals with VMs (Table 1) but one individual (Proband 4) had two KIAA1217 variants located in exon 13 that were three residues apart, shown to be in trans with each being inherited from a different parent. In Probands 1, 4, and 10 the variants were inherited from an unaffected parent and in the other seven cases, the parents’ samples were not available for segregation analysis.
Probands’ 4 and 5 KIAA1217 variants are predicted to be “definitely damaging” and probands’ 2, 7, and 10 variants are predicted to be possibly damaging based on their HVAR and CADD scores. Given the low HVAR scores for the p.A145T and p.I1293V variants seen in patients 1 and 8, respectively, we believe caution should be expressed when interpreting their potential significance.
KIAA1217 (sickle tail protein homolog) has 21 exons and encodes a 1943 amino acid protein product with Coiled-coil regions and Actin interacting domains (Figure 2). The coded protein is required for normal development of intervertebral disc, the regulation of dendritic spine morphogenesis, embryonic skeletal system development through regulating cell migration, multicellular organism development, and substrate adhesion-dependent cell spreading (The Gene Ontology Project). KIAA1217 has been previously linked to genetic susceptibility to intervertebral disc degeneration and lumbar disc herniation (Karasugi et al., 2009; Kelempisioti et al., 2011). It is possible that pathogenic variants in KIAA1217 may contribute to the development of VMs through perturbation of intervertebral disc development. Animal model studies showed that mice homozygous for a gene-trapped allele display malformations of the notochord and caudal vertebrae and may exhibit caudal tail kinks. Mice homozygous for another gene-trapped allele have malformed caudal vertebrae and intervertebral disk abnormalities and about half display kinked tails (KIAA1217, 2020).
In addition, at DECIPHER, there are 13 individuals with large deletions that disrupt KIAA1217 among other genes. Six of the deletions are classified as pathogenic or likely pathogenic and none of them is classified as benign or likely benign. Twelve of them are in individuals with an abnormal phenotype, eight of which have skeletal abnormalities (hemivertebrae, hypoplasia of the radius, short stature, postnatal growth retardation, camptodactyly of finger, hammertoe, hip dislocation, short toe, abnormality of the hand, arachnodactyly, finger clinodactyly, and short neck). A heterozygous, de novo, 10.44 Mb (97 genes) deletion in a proband with hemivertebrae, abnormal shaped pinna, radial hypoplasia, low set ears, and renal agenesis is one of these cases (DECIPHER ID 248253). Another is a heterozygous, de novo, 17.4 Mb (179 genes) deletion classified as likely pathogenic in an individual with 2–3 toe syndactyly, abnormality of the pinna, anteverted nares, brachycephaly, delayed speech and language development, finger clinodactyly, high palate, intellectual disability, low-set ears, muscular hypotonia, narrow mouth, prominent nasal bridge, short neck, and short philtrum (DECIPHER ID 397158) (https://decipher.sanger.ac.uk/patient/248253#genotype/cnv/18543/browser). These two deletions more specifically suggest the role of KIAA1217 loss of function variants in the etiology of VMs.
In our cohort of individuals with KIAA1217 variants, other phenotypic features include tethered cord and ASD/VSD (Table 1). Because the spinal cord develops simultaneously with the somite-derived musculoskeletal portions of the spine, disruption of one element would be expected to impact the development of other (Erol et al., 2004).
In Probands 1 and 10, the heterozygous variants were found to be inherited from an unaffected parent. Some of the explanations for this incomplete penetrance finding are (a) A mild phenotype not recognized in an apparently unaffected parent; (b) A mutant allele acting in concert with environmental factors; (c) An autosomal recessive phenotype where the affected individual has a second coding or noncoding variant in KIAA1217 that was not yet identified; or, (d) An oligogenic phenotype where the affected individual has a second coding or noncoding variant in a different gene that was not yet recognized. A similar situation has been described before in association with VMs. A heterozygous c.1013C > T missense allele in T (brachyury) has been identified in three unrelated individuals with VMs including two patients with cervical fusion defects and one patient with sacral agenesis. In each case, a clinically asymptomatic parent carried the mutant allele (Ghebranious et al., 2008). The identical variant was identified in another patient with sacral agenesis and his/her clinically unaffected parent reported by Papapetrou et al. (1999). Heterozygous mutant adult T (brachyury) mice have a short tail phenotype. Homozygous mutant T mice die at embryonic Day 10 due to the presence of an allantoic bladder (Edwards et al., 1996).
In summary, we identified 11 KIAA1217 rare variants in 10 probands, three of which have not been described in gnomAD and one of which is a nonsense variant. Based on our findings and what is known about KIAA1217 function in humans and mouse model, we suggest that the KIAA1217 variants identified here are strong causative candidates for the VM phenotypes described in the probands in our cohort and that pathogenic, loss-of-function variants in this gene may cause autosomal recessive or dominant with incomplete penetrance VM with or without heart and central nervous system anomalies or other anomalies. Future studies in larger cohorts and functional studies of the candidate variants identified in our cohort are necessary to confirm the causal relationship between KIAA1217 and VMs. Additionally, continued collaborative efforts between developmental biologists, molecular geneticists, and clinicians are instrumental to achieve a better understanding of the intricacies of somitogenesis and its application to clinical settings.
Supplementary Material
ACKNOWLEDGMENTS
This project was partially funded by the Baylor-Hopkins Center for Mendelian Genomics (NHGRI UM1 HG006542), National Natural Science Foundation of China (81822030 to N.W., 81772299 to Z.W., and 81772301 to G.Q.), National Institutes of Health (R03HD099516) to P.F.G., the CAMS Initiative Fund for Medical Sciences (2016-I2M-3-003 to G.Q. and N.W., 2016-I2M-2-006 to Z.W., 2017-I2M-2-001 to Z.W.), and the National Key Research and Development Program of China (No. 2018YFC0910500). Marshfield Clinic, University of Wisconsin-Madison Pediatrics Department, Scoliosis Research Society. This work was supported by the National Health and Medical Research Council (NHMRC) Project Grant ID 635500 and Fellowships ID514900, ID1042002 to S.L.D.
Funding information
National Human Genome Research Institute, Grant/Award Number: NHGRI UM1 HG006542; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R03HD099516; National Key and Development Program of China, Grant/Award Number: 2018YFC0910500; National Natural Science Foundation of China, Grant/Award Numbers: 81822030, 81772299, 81772301; NHMRC, Grant/Award Numbers: 635500, ID514900, ID1042002; The CAMS Initiative Fund for Medical Sciences, Grant/Award Numbers: 2016-I2M-3-003, 2016-I2M-2-006, 2017-I2M-2-001
CONFLICT OF INTEREST
R.D.S. declares equity interest in and consulting fees received from Acer Therapeutics and Censa Pharmaceuticals, and consulting fees from Retrophin. He is the principal investigator of an investigator-initiated observational research study funded by Alexion via a contract with Marshfield Clinic Health System. In the past 5 years, he has received travel support from Pfizer for a meeting to review clinical trial results, and consulting fees from Raptor, Biomarin, Alexion, E-Scape Bio, Health Advances, Precision for Value, and Best Doctors. R.D.S. is an employee of Prevention Genetics, which was not involved in this study. The rest of the authors declare no conflict of interest.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Al Mutairi F, Alzahrani F, Ababneh F, Kashgari AA, & Alkuraya FS (2018). A Mendelian form of neural tube defect caused by a de novo null variant in SMARCC1 in an identical twin. Annals of Neurology, 83(2), 433–436. 10.1002/ana.25152 [DOI] [PubMed] [Google Scholar]
- Alazami AM, Kentab AY, Faqeih E, Mohamed JY, Alkhalidi H, Hijazi H, & Alkuraya FS (2015). A novel syndrome of Klippel-Feil anomaly, myopathy, and characteristic facies is linked to a null mutation in MYO18B. Journal of Medical Genetics, 52(6), 400–404. 10.1136/jmedgenet-2014-102964 [DOI] [PubMed] [Google Scholar]
- Bayrakli F, Guclu B, Yakicier C, Balaban H, Kartal U, Erguner B, … Kars HZ (2013). Mutation in MEOX1 gene causes a recessive Klippel-Feil syndrome subtype. BMC Genetics, 14(1), 1. 10.1186/1471-2156-14-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard-Lacroix E, Tardif J, Camurri MV, Lemyre E, Barchi S, Parent S, & Campeau PM (2017). Retrospective analysis of congenital scoliosis: associated anomalies and genetic diagnoses. Spine, 42 (14), E841–E847. [DOI] [PubMed] [Google Scholar]
- Chen W, Lin J, Wang L, Li X, Zhao S, Liu J, … Zha WN (2020). TBX6 missense variants expand the mutational spectrum in a non-Mendelian inheritance disease. Human Mutation, 41(1), 182–195. 10.1002/humu.23907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu Z, Chen J, Zuo Y, Liu S, Chen W, … Wu Z (2016). The genetic landscape and clinical implications of vertebral anomalies in VACTERL association. Journal of Medical Genetics, 53(7), 431–437. 10.1136/jmedgenet-2015-103554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depristo MA, Banks E, Poplin RE, Garimella KV, Maguire JR, Hartl C, … Simches RB (2011). A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics, 43(5), 491–498. 10.1038/ng.806.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequéant M-L, Glynn E, Gaudenz K, Wahl M, Chen J, Pourquié O, … Pourquié O (2006). A complex oscillating genes network of signaling underlies clock the mouse segmentation. Science, 314(5805), 1595–1598. 10.1126/science.1133141 [DOI] [PubMed] [Google Scholar]
- Eckalbar WL, Fisher RE, Rawls A, & Kusumi K (2012). Scoliosis and segmentation defects of the vertebrae. Wiley Interdisciplinary Reviews: Developmental Biology, 1(3), 401–423. 10.1002/wdev.34 [DOI] [PubMed] [Google Scholar]
- Edwards YH, Putt W, Lekoape KM, Stott D, Fox M, Hopkinson DA, & Sowden J (1996). The human homolog T of the mouse T (grachyury) gene; gene structure, cDNA sequence, and assignment to chromosome 6q27. Genome Research, 6(3), 226–233. [DOI] [PubMed] [Google Scholar]
- Erol B, Tracy MR, Dormans JP, Zackai EH, Maisenbacher MK, O’Brien ML, … Kusumi K (2004). Congenital scoliosis and vertebral malformations: Characterization of segmental defects for genetic analysis. Journal of Pediatric Orthopaedics, 24(6), 674–682. 10.1097/01241398-200411000-00015 [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Blank RD, Raggio CL, Staubli J, Mcpherson E, Ivacic L, … Giampietro PF (2008). A missense T (Brachyury) mutation contributes to vertebral malformations. Joournal of Bone and Mineral Research, 23(10), 1576–1583. 10.1359/JBMR.080503 [DOI] [PubMed] [Google Scholar]
- Giampietro PF, Dunwoodie SL, Kusumi K, Pourquié O, Tassy O, Offiah AC, … Turnpenny PD (2009). Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Annals of the New York Academy of Sciences, 1151, 38–67. 10.1111/j.1749-6632.2008.03452.x [DOI] [PubMed] [Google Scholar]
- Hubaud A, & Pourquié O (2014). Signalling dynamics in vertebrate segmentation. Nature Reviews Molecular Cell Biology, 15(11), 709–721. 10.1038/nrm3891 [DOI] [PubMed] [Google Scholar]
- Karaca E, Yuregir OO, Bozdogan ST, Aslan H, Pehlivan D, Jhangiani SN, … Baylor-Hopkins Center for Mendelian Genomics. (2015). Rare variants in the notch signaling pathway describe a novel type of autosomal recessive Klippel-Feil syndrome. American Journal of Medical Genetics, Part A, 167A(11), 2795–2799. 10.1002/ajmg.a.37263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasugi T, Semba K, Hirose Y, Kelempisioti A, Nakajima M, Miyake A, … Ikegawa S (2009). Association of the tag SNPs in the human SKT gene (KIAA1217) with lumbar disc herniation. Journal of Bone and Mineral Research, 24(9), 1537–1543. 10.1359/jbmr.090314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelempisioti A, Eskola PJ, Okuloff A, Karjalainen U, Takatalo J, Daavittila I, … Männikkö M (2011). Genetic susceptibility of intervertebral disc degeneration among young Finnish adults. BMC Medical Genetics, 12(1), 153. 10.1186/1471-2350-12-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIAA1217. (2020). Mouse phenotype data were retrieved from the Mouse Genome Database (MGD). Bar Harbor, Maine: Mouse Genome Informatics, The Jackson Laboratory. [Google Scholar]
- Liu J, Wu N, Yang N, Takeda K, Chen W, Li W, … Qiu G (2019). TBX6-associated congenital scoliosis (TACS) as a clinically distinguishable subtype of congenital scoliosis: Further evidence supporting the compound inheritance and TBX6 gene dosage model. Genetics in Medicine, 21(7), 1548–1558. 10.1038/s41436-018-0377-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughran JM, Oates A, Donnai D, Read AP, & Tassabehji M (2003). Mutations in PAX1 may be associated with Klipel-Feil syndrome. European Journal of Human Genetics, 11(6), 468–474. 10.1038/sj.ejhg.5200987 [DOI] [PubMed] [Google Scholar]
- Mittmann N, Stout NK, Lee P, Tosteson ANA, Trentham-Dietz A, Alagoz O, & Yaffe MJ (2015). Total cost-effectiveness of mammography screening strategies. Health Reports, 26(12), 16–25. 10.1002/nbm.3369.Three [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou C, Drummond F, Reardon W, Winter R, Spitz L, & Edwards YH (1999). A genetic study of the human T gene and its exclusion as a major candidate gene for sacral agenesis with anorectal atresia, 36(3), 208–213. [PMC free article] [PubMed] [Google Scholar]
- Patten SA, Jacobs-McDaniels NL, Zaouter C, Drapeau P, Albertson RC, & Moldovan F (2012). Role of Chd7 in zebrafish: A model for CHARGE syndrome. PLoS One, 7(2), e31650. 10.1371/journal.pone.0031650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobreira N, Boehm C, Valle D, & Hamosh A (2015). New tools for Mendelian disease gene identification: PhenoDB variant analysis module; and GeneMatcher, a web-based tool for linking investigators with an interest in the same gene. Human Mutation, 36(4), 425–431. 10.1002/humu.22769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow DB, Chapman G, Turnpenny PD, & Dunwoodie SL (2007). Disruption of the somitic molecular clock causes abnormal vertebral segmentation. Birth Defects Research Part C - Embryo Today: Reviews, 81(2), 93–110. 10.1002/bdrc.20093 [DOI] [PubMed] [Google Scholar]
- Szot JO, Cuny H, Blue GM, Humphreys DT, Ip E, Harrison K, … Dunwoodie SL (2018). A screening approach to identify clinically actionable variants causing congenital heart disease in exome data. Circulation: Genomic and Precision Medicine, 11(3), e001978. 10.1161/CIRCGEN.117.001978 [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Fang ZM, Hilton EN, McGaughran J, Zhao Z, de Bock CE, … Clarke RA (2008). Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Human Mutation, 29(8), 1017–1027. 10.1002/humu.20741 [DOI] [PubMed] [Google Scholar]
- Turnpenny PD, Alman B, Cornier AS, Giampietro PF, Offiah A, Tassy O, … Dunwoodie S (2007). Abnormal vertebral segmentation and the notch signaling pathway in man. Developmental Dynamics, 236 (6), 1456–1474. 10.1002/dvdy.21182 [DOI] [PubMed] [Google Scholar]
- van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Levy-moonshine A, Jordan T, … Depristo MA (2014). From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Current Protocols in Bioinformatics, 43, 11.10.1–11.10.33. 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhao S, Liu B, Zhang Q, Li Y, Liu J, … Wu N (2018). Perturbations of BMP/TGF-β and VEGF/VEGFR signalling pathways in non-syndromic sporadic brain arteriovenous malformations (BAVM). Journal of Medical Genetics, 55(10), 675–684. 10.1136/jmedgenet-2017-105224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, … Zhang F (2015). TBX6 null variants and a common hypomorphic allele in congenital scoliosis. The New England Journal of Medicine, 372(4), 341–350. 10.1039/b800799c.O [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Berry-Wynne KM, Asai-Coakwell M, Sundaresan P, Footz T, French CR, … Lehmann OJ (2010). Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Human Molecular Genetics, 19(2), 287–298. 10.1093/hmg/ddp496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.