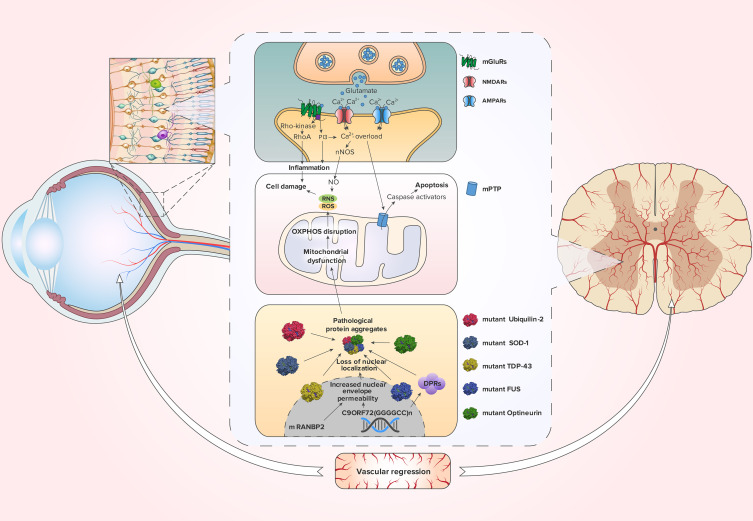
Figure 1.
Pathological pathways that retina cells can share with motor neurons in ALS.
Notes: Excessive release of glutamate leads to the stimulation of NMDARs, AMPARs and mGluRs. Activated NMDARs and AMPARs open up and create Ca2+-influx. Overload by Ca2+ causes mitochondrial permeability transition pore (mPTP) opening. As aresult, the mitochondrion swells, collapses, and releases caspase activators, which lead to caspase-dependent apoptosis.175,176,186 Additionally, activation of NMDARs is closely coupled to the generation of nitric oxide (NO) through neuronal nitric oxide synthase (nNOS),136 which promote reactive nitrogen species (RNS) and subsequent reactive oxygen species (ROS) production.177 Moreover, through mGluRs, glutamate activates RhoA and IP3, which stimulate inflammation and cell damage.187 ALS-associated mutations in SOD-1, Ubiquilin-2 and Optineurin, affecting their functions and causing protein aggregation, enhance mitochondrial dysfunction. Furthermore, mitochondrial dysfunction leads to the rupture of the oxidative phosphorylation (OXPHOS) process, resulting in the production of ROS. The same phenomenon occurs with the FUS proteins and TDP-43, which lose their nuclear localization with increasing nuclear permeability. The hexanucleotide expansion of the C9ORF72 gene and dysfunction of the RANBP2 protein contribute to the decrease in nuclear permeability. Dipeptide repeats (DPRs) that are encoded by disrupted C9ORF72 are also involved in aggregate formation. These processes can also be complicated by vascular regression.