Figure 2.
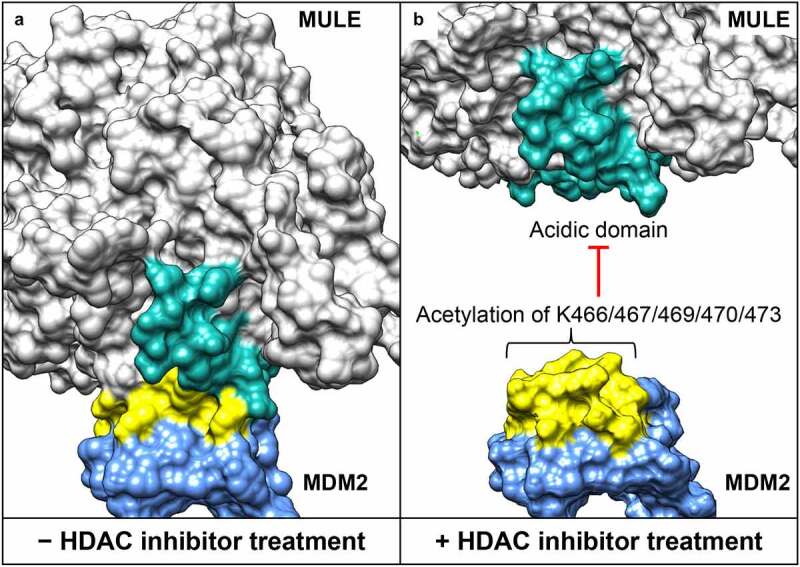
Structural interface between the acidic domain of Mcl-1 ubiquitin ligase E3 (MULE) and the lysine-rich domain of mouse double minute 2 homolog (MDM2). (a) Surface presentation of the MULE–MDM2 interaction. Models for MULE (residues 2261–2970) and MDM2 (residues 418-491) are generated by high-resolution comparative modeling with the Robetta server,39 and docking analysis performed using the ClusPro server.40 (b) Histone deacetylase (HDAC) inhibitor–induced lysine acetylation of MDM2 (at position 466, 467, 469, 470 and 473), leading to MULE dissociation. The acidic domain of MULE (residues 2432-2465) is highlighted in cyan, and the lysine–rich domain of MDM2 (residues 460-476) highlighted in yellow.
