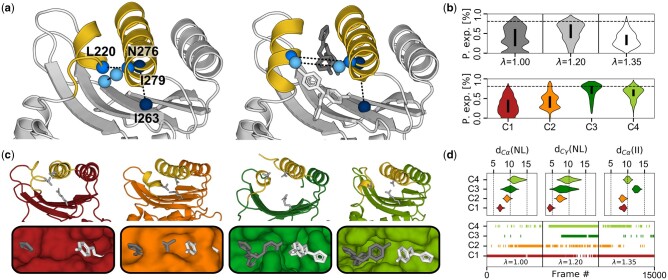
Fig. 4.
Identification of different opening states of the allosteric cryptic pocket in TEM1 β-lactamase. (a) apo and Holo structures (left and right, respectively). Allosteric inhibitors are shown in gray and white. Features following helical opening include the distance between Cα atoms of N276 and L220 (medium blue) and the Cγ of their sidechain (light blue). Pocket depth is monitored by the distance between Cα-carbons of I263 and 279 (dark blue). (b) The pocket exposure calculated using the fpocket software for the original replicas (top) and for each clusters (bottom). The dotted line in both is the reference value of the holo crystal structure used in the original paper. (c) The center of each cluster in cartoon representation on top of a surface representation of the allosteric pocket, highlighting the different states of helical openness and pocket depth. The triad N276-L220-R244 governing pocket opening and closing are shown as gray sticks. (c) The distribution of each feature for each cluster (top) and the cluster assignation along the three chosen replicas (bottom)