Abstract
Background:
Anti-mitochondrial antibodies (AMA) are found in >90% of primary biliary cholangitis patients. Anti-rods/rings antibodies (anti-RR) are most commonly associated with interferon-α and ribavirin treatment in hepatitis C patients. Clinical laboratories routinely screen for AMA and anti-RR using indirect immunofluorescence on HEp-2 cells (HEp-2-IFA). Therefore, we sought to establish reference materials for use in AMA and anti-RR testing.
Methods:
AMA-positive and anti-RR-positive human plasma samples (AMA-REF and RR-REF), identified as potential reference materials based on preliminary data, were further validated by multiple laboratories using HEp-2-IFA, immunoprecipitation (IP), western blotting, IP-western, line immunoassay (LIA), addressable laser bead immunoassay (ALBIA), and ELISA.
Results:
AMA-REF showed a strong positive cytoplasmic reticular/AMA staining pattern by HEp-2-IFA to ≥1:1280 dilution, and positive signal on rodent kidney/stomach/liver tissue. AMA-REF reacted with E2/E3, E3BP, E1α, and E1β subunits of the pyruvate dehydrogenase complex by IP and western blotting, and was positive for AMA antigens by LIA, ALBIA, and ELISA. RR-REF showed a strong positive rods and rings staining pattern by HEp-2-IFA to ≥1:1280 dilution. RR-REF reacted with inosine monophosphate dehydrogenase by IP, IP-western, and ALBIA. RR-REF also produced a nuclear homogenous staining pattern by HEp-2-IFA, immunoprecipitated proteins associated with anti-U1RNP antibody, and reacted weakly with histones, nucleosomes, Sm, and nRNP/Sm by LIA.
Conclusions:
AMA-REF and RR-REF are useful reference materials for academic or commercial clinical laboratories to calibrate and establish internal reference standards for immunodiagnostic assays. AMA-REF and RR-REF are now available for free distribution to qualified laboratories through Plasma Services Group.
Keywords: anti-mitochondrial antibody, anti-rods/rings antibody, autoantibody, autoimmunity, hepatitis C, primary biliary cholangitis
Introduction
Autoantibodies targeting self-antigens are hallmarks of many systemic and organ-specific autoimmune diseases. In a clinical laboratory setting, autoantibodies against nuclear and some cytoplasmic antigens are routinely screened by the indirect immunofluorescence assay (IFA) on HEp-2 cells (HEp-2-IFA). Several multiplex assays, using methodological platforms such as the enzyme-linked immunosorbent assay (ELISA), chemiluminescence immunoassay (CLIA), line immunoassay (LIA), addressable laser bead immunoassay (ALBIA), and new chip-based assays are becoming more common in diagnostic laboratories. However, HEp-2-IFA remains the “gold standard” method for detection of antinuclear antibodies (ANA) (1). ANA, which bind components of the nucleus, have been the focus of study for decades, but many autoantibodies that target cytoplasmic antigens are detected by HEp-2-IFA. One example is anti-mitochondrial antibodies (AMA), which target proteins of the inner and outer mitochondrial membranes. AMA are classified into 9 distinct categories, referred to as M1 through M9 subtypes (2, 3). AMA-M2 target the E2 components of the 2-oxo-acid dehydrogenase family of enzyme complexes, including the pyruvate dehydrogenase complex (PDC-E2), branched-chain 2-oxo-acid dehydrogenase complex (BCOADC-E2), and 2-oxo-glutarate dehydrogenase complex (OGDC-E2), typically referred to as the major AMA antigens (4). AMA-M2 also target the minor antigens dihydrolipoamide dehydrogenase (E3)-binding protein (E3BP) and the E1α subunit of the pyruvate dehydrogenase complex (PDC-E1α). AMA-M2 are highly specific for primary biliary cholangitis (PBC), formerly known as primary biliary cirrhosis (5, 6), a relatively uncommon chronic autoimmune disease resulting in progressive destruction of the intrahepatic biliary tree that can eventually lead to liver cirrhosis. Approximately 95% of PBC patients are AMA-positive, with PDC-E2 being the main antigen, and circulating AMA-M2 is one of three key diagnostic criteria for this disease (6–8). As the presence of AMA often precedes the clinical manifestation of PBC, accurate and complete reporting of AMA in AMA-specific assays and HEp-2-IFA is critical to enabling early diagnosis and treatment of PBC (9–11).
Another example of an autoantibody to cytoplasmic components is anti-rods/rings antibody (anti-RR). Unlike AMA, the study of anti-RR has uncovered only one major autoantigen, inosine 5′-monophosphate dehydrogenase 2 (IMPDH2) (12–14). IMPDH1 might also be recognized by anti-RR, considering the 84% sequence similarity between the two isoforms. Some human sera positive for anti-RR by HEp-2-IFA do not immunoprecipitate IMPDH, suggesting there are other antigens yet to be identified (15). Despite reports that cytidine triphosphate synthase (CTPS) co-localizes with IMPDH in rods/rings structures under certain conditions (12, 16, 17), no human anti-RR serum has been found to react with CTPS (13, 15). Several reports showed that anti-RR is strongly associated with hepatitis C virus (HCV) after treatment with interferon-α and ribavirin (IFN/RBV) therapy (13, 18–22). However, anti-RR has been observed occasionally in individuals without HCV infection (23), and in rare cases of systemic lupus erythematosus (24) and hepatitis B (19). Low-titer anti-RR has also been reported in the general population of the United States (24). Although the use of IFN/RBV therapy will likely decrease with the recent development of novel direct-acting antivirals (DAA), many DAA-based treatment regimens still include RBV (25, 26). IFN/RBV therapy might also continue in parts of the world where the considerable cost of new DAA prevent their rapid adoption (27). Considering how much is still unknown about anti-RR antibody, it will be useful to continue to accurately monitor and report its presence during routine ANA testing in clinical laboratories.
In this report, we describe the development of reference materials for the detection of AMA and anti-RR autoantibodies, which we refer to here as the AMA reference material (AMA-REF) and anti-rods/rings reference material (RR-REF). AMA-REF and RR-REF can be used to calibrate and establish internal reference standards for daily use by academic or commercial clinical laboratories performing HEp-2-IFA, immunoprecipitation (IP), or other immunodiagnostic assays, including western blot, ELISA, CLIA, LIA, and ALBIA.
Materials and Methods
Patient information and reference sample preparation
AMA-REF and RR-REF sera were collected by Plasma Services Group (PSG, Huntingdon Valley, PA, USA) from single donors. The AMA-REF donor was a 65-year-old Caucasian woman diagnosed with PBC. The RR-REF donor was a 47-year-old African-American woman diagnosed with systemic lupus erythematosus, but is anti-dsDNA-negative, HCV-negative, and has never received ribavirin or interferon. Both samples were prepared by PSG as defibrinated human plasma, undiluted without preservative.
Ethical approval
Research using only de-identified human samples in this study complies with all relevant national regulations and institutional policies. Informed consent was obtained by PSG in the collection of reference materials and is approved by appropriate institutional review boards.
HEp-2 indirect immunofluorescence assay (HEp-2-IFA)
HEp-2-IFA was performed on AMA-REF, RR-REF, and positive control sera using HEp-2 cell substrate from Inova Diagnostics (San Diego, CA, USA), Bio-Rad (Hercules, CA, USA), or AESKU.Diagnostics GmbH (Wendelsheim, Germany) as previously described (28). AMA-REF and RR-REF were confirmed by expert technicians to produce characteristic staining patterns as designated by the International Consensus on ANA Patterns (ICAP) (29). AMA-REF and RR-REF were tested by two-fold serial dilution from a starting dilution of 1:40 to at least 1:1280, and were positive at 1:1280 even when using different secondary antibodies, which included: Alexa Fluor 488-conjugated AffiniPure F(ab′)2 fragment goat anti-human IgG, Fcγ fragment specific (109-546-098, Jackson ImmunoResearch, West Grove, PA, USA) diluted 1:400 in PBS (used in Figures 1 and 2); Alexa Fluor 488-conjugated goat anti-human IgG (A11013, Thermo Fisher Scientific, Waltham, MA, USA) diluted 1:400 in PBS; and FITC IgG conjugate with DAPI premixed solution, undiluted (508102, Inova Diagnostics). Fluorescent images were captured with a 40X objective manually or with the NOVA View automated microscopy system (Inova Diagnostics), which produced the same results for AMA-REF and RR-REF up to 1:1280 dilution. Images shown in Figures 1 and 2 were captured on an Olympus BX53 fluorescence microscope.
Figure 1. AMA-REF validation by HEp-2-IFA, radioimmunoprecipitation, and western blotting.
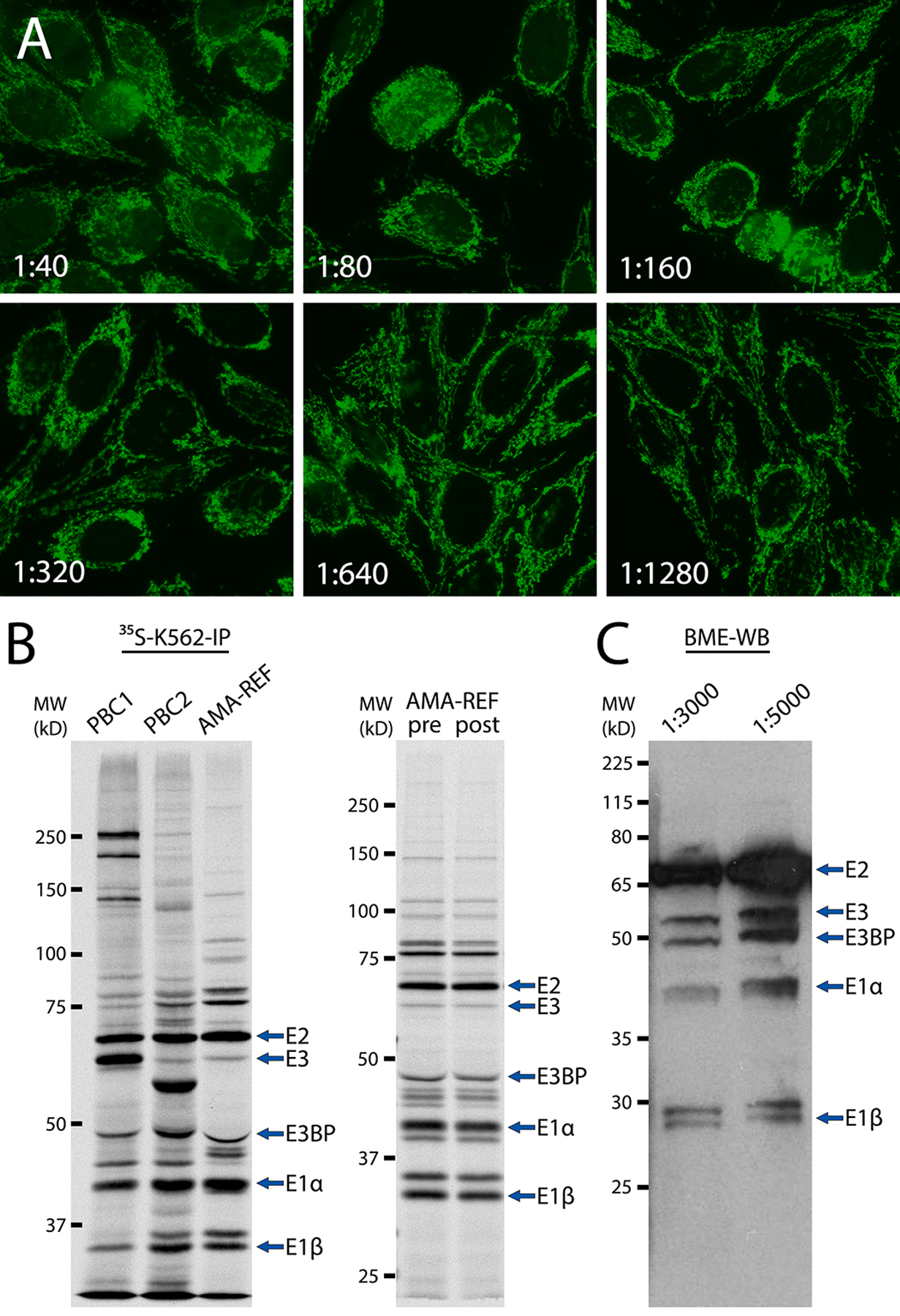
(A) AMA-REF serum was serially diluted from 1:40 to 1:1280 and analyzed by HEp-2-IFA, which showed a coarse granular filamentous staining pattern in the cytoplasm (ICAP AC-21) typical of known AMA sera. (B) Left panel: immunoprecipitation using 35S-methionine-labeled K562 cell extract (35S-K562-IP) of AMA-REF and AMA-positive control sera PBC1 and PBC2. Right panel: 35S-K562-IP of AMA-REF pre- and post-lyophilization. Immunoprecipitated proteins were subjected to SDS-PAGE in an 8.5% gel followed by autoradiography. Like PBC1 and PBC2, AMA-REF immunoprecipitated four protein subunits E2/E3, E3BP, E1α, and E1β of the pyruvate dehydrogenase complex (PDC), typical of AMA sera. (C) Western blotting of bovine mitochondrial extract (BME-WB) with AMA-REF diluted to 1:3000 or 1:5000 demonstrated reactivity with the same E2/E3, E3BP, E1α, and E1β PDC subunits.
Figure 2. RR-REF validation by HEp-2-IFA, radioimmunoprecipitation, and IP-western.
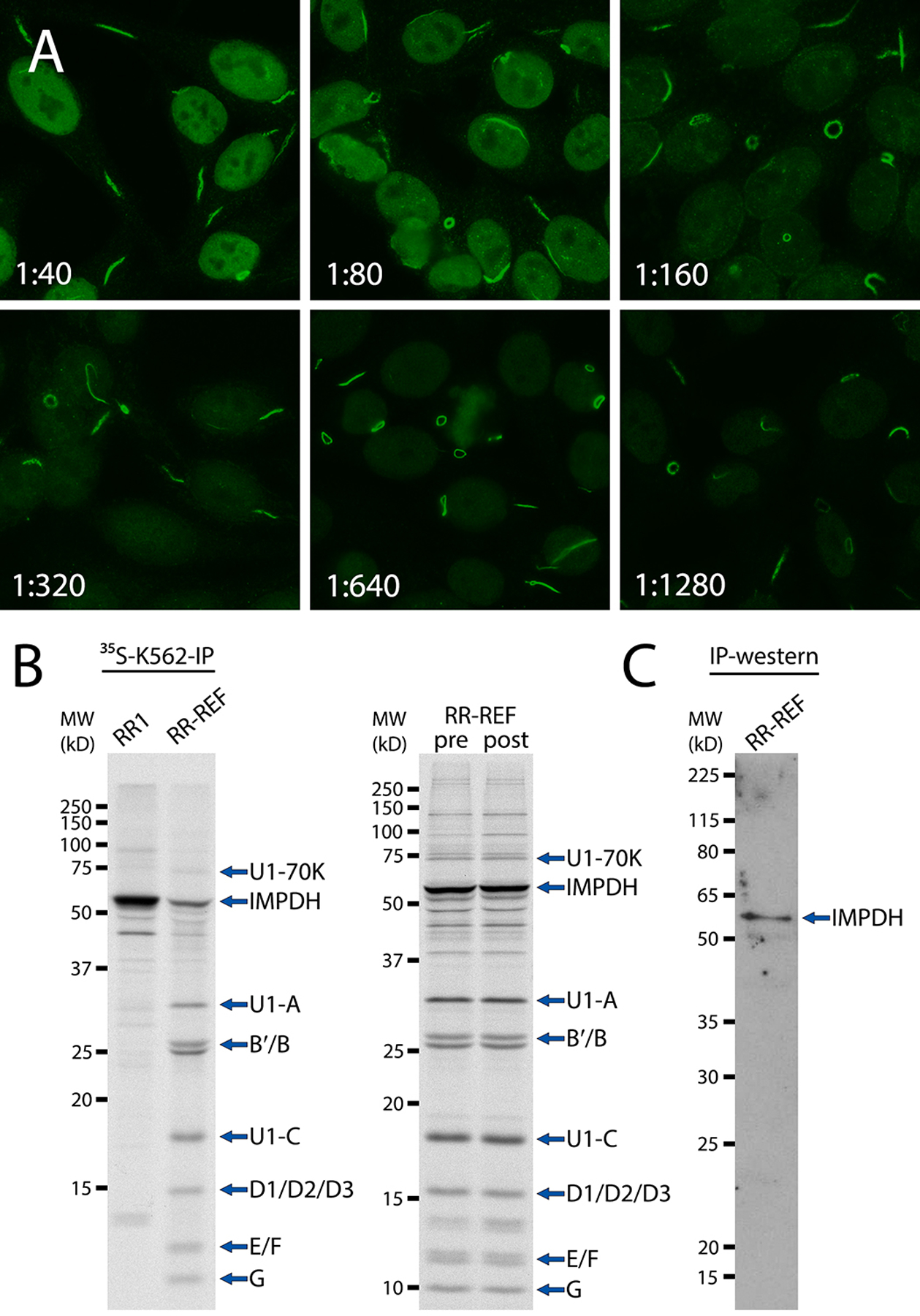
(A) RR-REF serum was serially diluted from 1:40 to 1:1280 and analyzed by HEp-2-IFA, which showed distinct rod- and ring-shaped structures, mainly in the cytoplasm of interphase cells, corresponding to the characteristic rods and rings staining pattern (ICAP AC-23). (B) Left panel: immunoprecipitation using 35S-methionine-labeled K562 cell extract (35S-K562-IP) of RR-REF and RR-positive control serum RR1. Right panel: 35S-K562-IP of RR-REF pre- and post-lyophilization. Immunoprecipitated proteins were subjected to SDS-PAGE in a 13% gel followed by autoradiography. Like RR1, RR-REF immunoprecipitated ~56 kD inosine 5′-monophosphate dehydrogenase (IMPDH). RR-REF also immunoprecipitated proteins associated with anti-U1RNP antibody, including the U1–70K, U1-A, and U1-C proteins, as well as Sm core proteins B′/B, D1/D2/D3, E, F, and G. (C) Immunoprecipitation of unlabeled MOLT-4 cell extract with RR-REF followed by western blotting (IP-western) with affinity-purified rabbit polyclonal anti-IMPDH1 antibody. A ~56 kD band corresponding to IMPDH was visible with no other bands detected, confirming reactivity of RR-REF with IMPDH.
Radioimmunoprecipitation assay
Antigens recognized by AMA-REF, RR-REF, and positive control sera were analyzed by IP of radiolabeled K562 (human erythroleukemia) cell extract and SDS-PAGE as previously described (30–32). Briefly, cells were labeled for 14 hours with 4.2 mCi in 45 ml 35S-L-methionine and 35S-L-cysteine (NEG072, PerkinElmer, Waltham, MA, USA) and lysed in 0.5 M NaCl NET/IGEPAL CA-630 buffer (500 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl pH 7.5, 0.3% IGEPAL CA-630) containing 0.5 mM PMSF and 0.3 TIU/ml aprotinin. Cell extract was cleared by centrifugation and immunoprecipitated on Protein A Sepharose beads (17-0780-01, GE Healthcare, Marlborough, MA, USA) coated with antibodies from 8 μL of human serum. Beads were then washed with 0.5 M NaCl NET/IGEPAL CA-630 buffer. Immunoprecipitated proteins were subjected to SDS-PAGE followed by autoradiography.
AMA-REF western blotting
25 μg of mitochondrial extract from bovine heart tissue lysate (ab110338, Abcam, Cambridge, MA, USA) was subjected to 10% SDS-PAGE and transferred to nitrocellulose membrane. After blocking for 1 h in 5% non-fat dry milk, the membrane was incubated with AMA-REF (diluted at 1:3000 or 1:5000) for 1 h at room temperature, followed by washing in PBS with 0.05% Tween 20 before incubation with goat F(ab’)2 anti-human IgG conjugated to horseradish peroxidase (HRP) (2042–05, SouthernBiotech, Birmingham, AL, USA) at 1:10,000 dilution for 1 h at room temperature. Reactivity of AMA-REF was detected by SuperSignal West Pico PLUS chemiluminescent substrate (34577, Thermo Fisher).
IP-western analysis of RR-REF
MOLT-4 (human acute lymphoblastic leukemia) cells (1 × 109) were lysed with 2 mL Buffer A (150 mM NaCl, 10 mM Tris-HCl pH 7.5, 1.5 mM MgCl2, 0.5% NP-40) on ice for 15 min and centrifuged for 15 min at 4°C to obtain cell lysate. IP was performed using 5 μL RR-REF serum, 100 μL cell lysate, and 50 μL Dynabeads Protein A (10001D, Thermo Fisher) per manufacturer’s instructions. Immunoprecipitated proteins were subjected to 10% SDS-PAGE followed by immunoblotting with affinity-purified rabbit polyclonal anti-IMPDH1 antibody (22092–1-AP, Proteintech, Chicago, IL, USA) at 1:1000 dilution and HRP-conjugated goat anti-rabbit IgG (4050–05, SouthernBiotech) at 1:5000 dilution.
Line immunoassay (LIA), addressable laser bead immunoassay (ALBIA), and enzyme-linked immunosorbent assay (ELISA)
Aliquots of the reference materials were tested for AMA (M2, M2–3E) and other autoimmune liver disease-related autoantibodies (sp100, LKM-1, SLA/LP, gp210, Ro52/TRIM21) by LIA (EUROLINE Autoimmune Liver Diseases Profile, Euroimmun, Lübeck, Germany) and ALBIA (Inova Diagnostics) using kits and protocols provided by the manufacturers. The cutoffs for ALBIA were set at 3 standard deviations above the mean of control samples (100 median fluorescence intensity, MFI). RR-REF was tested for antibodies to IMPDH2 by utilizing the full-length human protein (Abnova, Taipei City, Taiwan) covalently coupled to addressable laser beads (Luminex Corporation, Austin, TX, USA) analyzed on a Luminex 200 fluorometer as previously described (21). ELISA for PDC-E2 was performed using an in-house protocol at Fleury Medicine and Health Laboratories.
Results
Validation of AMA-REF and RR-REF as appropriate reference materials was performed by nine laboratories affiliated with the Autoantibody Standardization Committee of the International Union of Immunological Societies (www.AutoAb.org). These nine laboratories acquired consistent results and a consensus was reached that these are appropriate reference materials for detection of AMA and anti-rods/rings. Data reported here are representative of all data collected by these nine laboratories.
AMA-REF validation
AMA-REF was first validated using HEp-2-IFA by seven reference laboratories using HEp-2 cell substrate from Inova Diagnostics (San Diego, CA, USA), and all reported that AMA-REF had a strong positive cytoplasmic reticular/AMA staining pattern from 1:40 to 1:1280 dilution (Fig. 1A, representative images). While five laboratories tested the sample only to 1:1280, two groups reported positive AMA staining at 1:2560 and 1:5120. Additionally, none of the seven laboratories reported any other staining patterns produced by this sample. One other reference laboratory that uses HEp-2 cell substrate from AESKU.Diagnostics GmbH (Wendelsheim, Germany) also tested AMA-REF and reported similar results. In the case of AMA, HEp-2-IFA is often performed in addition to IFA on rat (or mouse) kidney, stomach, and liver tissue, the traditional method used to detect AMA (33). In kidney/stomach/liver slides, the typical AMA pattern shows coarse granular staining in the cytoplasm of distal renal tubules, gastric parietal cells, and hepatocytes. Aside from HEp-2-IFA, two laboratories also performed the kidney/stomach/liver assay and both determined that AMA-REF was positive for this characteristic AMA pattern at 1:1280 dilution (data not shown).
In addition to IFA, one reference laboratory utilized IP to determine antigens recognized by AMA-REF. Using 35S-methionine-labeled K562 cell extract, AMA-REF immunoprecipitated protein bands corresponding to the four subunits E2/E3, E3BP, E1α, and E1β of the pyruvate dehydrogenase complex (PDC) recognized by AMA, which were clearly detected in the AMA positive control sera PBC1 and PBC2 (Fig. 1B, left panel) (32, 34, 35). Ceribelli et al. were recently the first to describe IP band patterns for AMA sera (32), identical to the bands we observed for AMA-REF. After initial validation that AMA-REF produced the correct IFA pattern and recognized typical AMA antigens, PSG prepared prototype vials of lyophilized AMA-REF and distributed them to eight reference laboratories to demonstrate the lyophilization, packaging, and distribution process for these sera. AMA-REF post-lyophilization was re-tested by IFA and all results were consistent with our first round of testing (data not shown). Additionally, IP of AMA-REF pre- and post-lyophilization produced identical band patterns (Fig. 1B, right panel). Western blotting of bovine mitochondrial extract with AMA-REF was also performed, at various dilutions ranging from 1:200 to 1:10,000. Characteristic bands associated with major PDC antigens were observed from 1:1000 to 1:5000 dilutions, with 1:3000 and 1:5000 showing the best signal-to-noise (Fig. 1C).
We then analyzed AMA-REF with additional immunoassays, such as LIA, ALBIA, and ELISA. Two laboratories performed LIA to determine reactivity of AMA-REF to highly purified antigens associated with autoimmune liver diseases (EUROLINE Autoimmune Liver Diseases Profile, Euroimmun, Lübeck, Germany). By LIA, AMA-REF recognized AMA-M2 and M2–3E (PDC-E2, BCOADC-E2, and OGDC-E2) antigens, but was negative for other autoantibodies associated with autoimmune liver disease, such as antibodies to sp100, PML, gp210, LKM-1, LC-1, and SLA/LP (Table 1). AMA-REF was also negative on LIA containing common autoantigens, such as Sm, Scl-70, Jo-1, Ro52, and others. When tested by ALBIA, AMA-REF had a strong positive reactivity with M2–3E with median fluorescence intensity (MFI) of 4569 and weak positive reactivity with sp100 (MFI: 561). AMA-REF did not react with gp210, LKM-1, LC-1, SLA/LP, HK, KL, and VCP antigens by ALBIA. Finally, AMA-REF was also positive by ELISA for reactivity with PDC-E2, the most common AMA antigen, with an absorbance value of 6.5 compared to a cutoff of 0.9.
Table 1.
Additional immunoassay data for AMA-REF.
| Assay | Positive antigens | Negative antigens |
|---|---|---|
| LIA | M2–3E* | sp100, PML, gp210, LKM-1, LC-1, SLA/LP, SSA/Ro60, SSA/Ro52, Scl70, CENP-A, CENP-B, PGDH |
| ALBIA | M2–3E, sp100 | LKM-1, SLA/LP, LC-1, HK, KL, gp210, VCP |
| ELISA | PDC-E2 |
3E/M2 includes PDC-E2, BCOADC-E2, and OGDC-E2 antigens
RR-REF validation
RR-REF was validated first using HEp-2-IFA by six laboratories using HEp-2 substrate from Inova Diagnostics. All laboratories reported that RR-REF showed a strong positive rods and rings staining pattern from 1:40 to 1:1280 dilution (Fig. 2A, representative images). While five laboratories tested the sample only to 1:1280, one group reported positive rods and rings staining at 1:5120. In addition to the rods and rings pattern, RR-REF also produced the nuclear homogenous pattern (ICAP AC-1) in HEp-2 cells at 1:40 and 1:80 dilutions. The coexisting AC-1 pattern may serve as an internal positive control for detection of anti-RR, as the antigenic rods/rings structures appear in cells only under certain culture conditions (12, 16, 36, 37). It follows that if rods/rings structures are not present in cells used as substrate, anti-RR antibodies cannot be detected. To date, in our hands, only HEp-2 cells from Inova Diagnostics and Euroimmun allow for consistent detection of anti-RR. Two laboratories tested RR-REF on HEp-2 cells from Bio-Rad (Hercules, CA, USA) and AESKU.Diagnostics GmbH (Wendelsheim, Germany). They also reported positive AC-1 pattern at low dilutions, but were unable to detect the rods/rings pattern, indicating that these substrates are not suitable for anti-RR testing. Thus, this additional AC-1 pattern is an important aspect of the utility of RR-REF as a reference serum for detection of anti-RR.
As IMPDH is the only known antigen of anti-RR, one reference laboratory also analyzed the reactivity of RR-REF with IMPDH by IP. RR-REF immunoprecipitated a ~56 kD protein corresponding to IMPDH (Fig. 2B, left panel), similar to anti-RR positive control serum RR1 and known anti-RR positive samples (38). RR-REF also immunoprecipitated proteins associated with anti-U1RNP antibody, including the U1–70K, U1-A, and U1-C proteins, as well as Sm core proteins B′/B, D1/D2/D3, E, F, and G (30, 39). We then tested RR-REF pre- and post-lyophilization by IFA and IP. Both assays demonstrated that lyophilization and distribution of RR-REF did not affect its reactivity (IP shown in Fig. 1B, right panel). To further confirm reactivity of RR-REF with IMPDH, we performed an IP-western blot procedure using unlabeled MOLT-4 cell lysate. Proteins immunoprecipitated by RR-REF were subjected to 10% SDS-PAGE followed by immunoblotting with purified anti-IMPDH antibody, which showed positive reactivity with a 56 kD protein corresponding to IMPDH (Fig. 2C).
RR-REF reactivity was then analyzed by LIA and ALBIA in a similar manner to AMA-REF. Although IMPDH is not an antigen included in commercial LIA assays from Euroimmun, we wanted to determine additional reactivity of this serum, considering its nuclear homogenous pattern by IFA and reactivity with anti-U1RNP proteins by IP. By LIA using the ANA Profile 3 from Euroimmun, RR-REF reacted positively with histones and nucleosomes and had a weak positive signal with Sm and nRNP/Sm antigens, and was negative for all other antigens (Table 2). By ALBIA, RR-REF reacted strongly with IMPDH2, but was negative for other antigens included in an extractable nuclear antigen (ENA) panel (Jo-1, Sm, nRNP/Sm, Ro52, Ro60, SSB, Pm/Scl, PCNA, CENPB, ribosome) and cytodots panel (GW182, GE-1, Ago2, LAMP2, EEA1, elastase).
Table 2.
Additional immunoassay data for RR-REF.
| Assay | Positive antigens | Negative antigens |
|---|---|---|
| LIA | Histones, nucleosomes, Sm, nRNP/Sm | DFS70 |
| ALBIA | IMPDH2 | GW182, GE-1, Ago2, LAMP2, EEA1, elastase, Jo-1, ribosome, Sm, Sm/RNP, Ro52, Ro60, SSB, PM/Scl, PCNA, CENP-B |
Discussion
Detection of serum autoantibodies is a crucial factor in the diagnosis of many autoimmune disorders. Clinicians rely on the accurate and timely detection and quantification of autoantibodies in patients suspected to have an autoimmune disorder. Thus, there have been widespread efforts to either standardize current detection methods or develop novel immunoassays to improve consistency in measurements of autoantibody production. Some high-throughput clinical laboratories have focused on automation to help reduce inter-laboratory variability. Several companies now offer automated HEp-2-IFA reading systems designed to reduce the subjectivity involved in analyzing certain HEp-2-IFA patterns by microscopy. In parallel, groups of experts, such as the Autoantibody Standardization Committee of the International Union of Immunological Societies (ASC, www.AutoAb.org), European Autoimmunity Standardization Initiative (EASI), and International Consensus on ANA Patterns (ICAP, www.ANApatterns.org), focus on developing guidelines, recommendations, and standard reference materials to improve interpretation of immunodiagnostic assays (29, 40–43). The use of reference sera to properly calibrate assays can allow quantitative measurements to be compared among laboratories.
The presence of circulating AMA-M2 is one of three major criteria for diagnosis of PBC (detectable in ~95% of PBC patients) (6–8) and often precedes clinical manifestations of the disease (9–11), highlighting the importance of accurate detection of AMA. However, significant variability in test results and discrepancies in reporting among different laboratories can affect proper diagnosis. It is therefore critical that we improve the consistency and accuracy in AMA testing across laboratories globally as much as possible. Aside from its high specificity for PBC, AMA-M2 can be detected in a low percentage of autoimmune hepatitis type 1 (44) and systemic sclerosis patients (32). Although these are often cases of overlapping PBC (45), the measurement of AMA is certainly relevant for patients suspected of having PBC, and may be critical in the prediction of PBC development for patients suffering from other autoimmune manifestations. In addition, the characteristic AMA-like AC-21 pattern can be detected by HEp-2-IFA in individuals under diagnosis workout for a variety of reasons (46).
Accordingly, one of our major goals as a group working towards standardization of autoantibody testing was the establishment of a reference serum for AMA that would be widely available to any laboratory seeking to set up internal standards for AMA detection. As our data show, AMA-REF consistently met expectations as a strong positive AMA reference material by IFA, IP, western blotting, LIA, ALBIA, and ELISA. IP and western blotting also demonstrated that AMA-REF contains antibodies that recognize antigens of the mitochondrial pyruvate dehydrogenase complex (E2, E3, E3BP, E1α, and E1β) typical of known AMA sera (5, 6, 32). Further examination by LIA and ALBIA confirmed reactivity with M2–3E antigens, which includes PDC-E2, BCOADC-E2, and OGDC-E2, but ALBIA also showed weak positive reactivity with sp100, which was not observed in other assays. The multiple nuclear dots pattern (ICAP AC-6) associated with sp100 was not detected by any laboratories in HEp-2-IFA. Taken together, data from seven different methods show that AMA-REF will be particularly useful for laboratories seeking to establish internal standards for the detection of AMA in a variety of immunoassays.
Although many anti-RR positive sera are HCV-positive, RR-REF is from an HCV-negative individual, facilitating its safe distribution to laboratories worldwide. In HEp-2-IFA, RR-REF produces the characteristic rods and rings staining pattern (ICAP AC-23), which shows distinct rod- and ring-shaped structures mainly in the cytoplasm of interphase cells (12, 18), with some structures visible in mitotic cells, and smaller structures evident in the nucleus (15). In addition to the rods and rings pattern, RR-REF shows a nuclear homogenous pattern at 1:40 and 1:80 dilutions. While HEp-2-IFA is the lone method capable of detecting anti-RR per se, we also evaluated RR-REF for its reactivity with inosine 5′-monophosphate dehydrogenase (IMPDH), the only known antigen of rods/rings structures. We demonstrated that RR-REF recognizes a ~56 kD protein corresponding to IMPDH by IP, typical of many known anti-RR positive sera (15, 38). We further validated this IMPDH reactivity by IP-western in MOLT-4 cell extract. ALBIA for IMPDH2 also confirmed this positive reactivity, while LIA showed additional reactivity to histones, nucleosomes, Sm, and nRNP/Sm. Altogether, these data suggest that RR-REF, especially with its internal control nuclear homogenous pattern, will be a useful tool as a reference material for anti-RR detection.
The clinical implications of anti-RR antibodies remain unclear. The rods and rings staining pattern, first described in the context of ANA patterns just under 10 years ago (47), is considered to be quite rare. However, it is not clear exactly how rare anti-RR antibodies are, due to the peculiarity that only HEp-2 substrate from select sources allow for consistent detection of rods/rings structures. Many patient sera positive for anti-RR may have been missed over the years due to being tested on substrate where immunoreactive rods/rings structures were not present. Additionally, anti-RR positivity does not appear to be useful as a marker for any autoimmune disorder or other types of disease, as the antibody tends to appear in HCV patients only after treatment with IFN/RBV (48–50). This interpretation is generally correct based on publications focused on anti-RR detection in HCV (13, 18–22), but seems not to be the complete story. For example, low-titer anti-RR were reported in 39 out of 4738 individuals in the National Health and Nutrition in the National Health and Nutrition Examination Survey, and 38 of these 39 individuals had no history of HCV (24). Climent et al. also recently reported 14 non-HCV cases of anti-RR that present other diseases, mainly of autoimmune origin (23). A third related point is that due to advancements in HCV treatment, it is likely that fewer patients will be treated with IFN/RBV in the future, thus making anti-RR even more rare. Still, many new treatment regimens continue to include RBV (25, 26) and are extremely costly, potentially necessitating the continued use of IFN/RBV in the developing world (27). Although the reasons behind this autoantibody production remain unclear, we recommend continued monitoring and reporting of anti-RR rather than ignoring the unknown.
In summary, aliquots of AMA-REF and RR-REF are available for free distribution (subjected to shipping charges) to all qualified academic or commercial clinical laboratories through Plasma Services Group (https://www.plasmaservicesgroup.com) as catalogue numbers IS2724 (AMA) and IS2725 (anti-RR).
Acknowledgments
The authors would like to thank Nice Carabellese, Franco Franceschini, Boris Gilburd, Haiyan Hou, Justin Nicholas, and Meifeng Zhang for their technical support.
Research funding
SJC is supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1315138, and was previously supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award No. 2T90DE021990-06 while this work was performed. MS is supported by JSPS KAKENHI (Grants-in-Aid for Scientific Research) grant number 15K08790. MJF is a co-investigator on the UCAN-CANDU project funded by the Canadian Institutes of Health Research. IGDLT receives support from the Mexican National Research System (SNI) from Conacyt (National Council of Science and Technology). CS is supported by the Italian Ministry of Foreign Affairs (PGR00807) and receives research funding from AESKU.Diagnostics GmbH (Wendelsheim, Germany) and Menarini Diagnostics (Florence, Italy). The IUIS Autoantibody Standardization Committee has received unrestricted grants from Bio-Rad, Inova Diagnostics, and Euroimmun. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
Honorarium
LECA has received speaking honoraria from Inova Diagnostics and Werfen International (Barcelona, Spain). MJF has received speaking honoraria from Inova Diagnostics (San Diego, CA, USA), Werfen International (Barcelona, Spain), Amgen Canada (Mississauga, ON, Canada), and Alexion Pharmaceuticals (New Haven, CT, USA). CS has received speaking honoraria from AESKU.Diagnostics GmbH, Menarini Diagnostics, and Grifols (Barcelona, Spain). AC and EKLC have also received speaking honoraria from Grifols.
List of Abbreviations:
- ALBIA
addressable laser bead immunoassay
- AMA
anti-mitochondrial antibodies
- AMA-REF
anti-mitochondrial antibody reference material
- ANA
antinuclear antibodies
- anti-RR
anti-rods/rings antibody
- ASC
Autoantibody Standardization Committee
- DAA
direct-acting antivirals
- ELISA
enzyme-linked immunosorbent assay
- HCV
hepatitis C virus
- ICAP
International Consensus on ANA Patterns
- IFA
indirect immunofluorescence assay
- IFN
interferon-α
- IMPDH
inosine 5′-monophosphate dehydrogenase
- LIA
line immunoassay
- PBC
primary biliary cholangitis
- PDC
pyruvate dehydrogenase complex
- PSG
Plasma Services Group
- RBV
ribavirin
- RR-REF
anti-rods/rings antibody reference material
Footnotes
Competing interests
MJF was and/or continues to be a consultant to Inova Diagnostics, Werfen International, Alexion Pharmaceuticals, and Bio-Rad (Hercules, CA, USA). CS is a consultant for AESKU.Diagnostics GmbH and Grifols. The funding organizations played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Employment or leadership
None declared.
References
- 1.Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010. August;69:1420–2. [DOI] [PubMed] [Google Scholar]
- 2.Berg PA, Klein R. Antimitochondrial antibodies in primary biliary cirrhosis and other disorders: definition and clinical relevance. Dig Dis. 1992;10:85–101. [DOI] [PubMed] [Google Scholar]
- 3.Berg PA, Klein R. Mitochondrial antigen/antibody systems in primary biliary cirrhosis: revisited. Liver. 1995. December;15:281–92. [DOI] [PubMed] [Google Scholar]
- 4.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, et al. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007. March;45:659–65. [DOI] [PubMed] [Google Scholar]
- 5.EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017. July;67:145–72. [DOI] [PubMed] [Google Scholar]
- 6.Shuai Z, Wang J, Badamagunta M, Choi J, Yang G, Zhang W, et al. The fingerprint of antimitochondrial antibodies and the etiology of primary biliary cholangitis. Hepatology. 2017. May;65:1670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev. 2014. Apr-May;13:441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005. September 22;353:1261–73. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta. 2011. March 18;412:502–12. [DOI] [PubMed] [Google Scholar]
- 10.Agmon-Levin N, Shapira Y, Selmi C, Barzilai O, Ram M, Szyper-Kravitz M, et al. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J Autoimmun. 2010. February;34:55–8. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet. 1996. November 23;348:1399–402. [DOI] [PubMed] [Google Scholar]
- 12.Carcamo WC, Satoh M, Kasahara H, Terada N, Hamazaki T, Chan JY, et al. Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cells. PLoS One. 2011;6:e29690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst C, Radzimski C, Blocker IM, Teegen B, Bogdanos DP, Stocker W, et al. Development of a recombinant cell-based indirect immunofluorescence assay (RC-IFA) for the determination of autoantibodies against “rings and rods”-associated inosine-5’-monophosphate dehydrogenase 2 in viral hepatitis C. Clin Chim Acta. 2013. March 15;418:91–6. [DOI] [PubMed] [Google Scholar]
- 14.Seelig HP, Appelhans H, Bauer O, Bluthner M, Hartung K, Schranz P, et al. Autoantibodies against inosine-5’-monophosphate dehydrogenase 2--characteristics and prevalence in patients with HCV-infection. Clin Lab. 2011;57:753–65. [PubMed] [Google Scholar]
- 15.Carcamo WC, Calise SJ, von Muehlen CA, Satoh M, Chan EKL. Molecular Cell Biology and Immunobiology of Mammalian Rod/Ring Structures. International Review of Cell and Molecular Biology, Vol 3082014. p. 35–74. [DOI] [PubMed] [Google Scholar]
- 16.Keppeke GD, John Calise S, Chan EKL, Andrade LEC. Assembly of IMPDH2-Based, CTPS-Based, and Mixed Rod/Ring Structures Is Dependent on Cell Type and Conditions of Induction. Journal of Genetics and Genomics. 2015. June 20 2015;42:287–99. [DOI] [PubMed] [Google Scholar]
- 17.Chang CC, Lin WC, Pai LM, Lee HS, Wu SC, Ding ST, et al. Cytoophidium assembly reflects upregulation of IMPDH activity. J Cell Sci. 2015. October 1;128:3550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covini G, Carcamo WC, Bredi E, von Muhlen CA, Colombo M, Chan EKL. Cytoplasmic rods and rings autoantibodies developed during pegylated interferon and ribavirin therapy in patients with chronic hepatitis C. Antivir Ther. 2012. December 1;17:805–11. [DOI] [PubMed] [Google Scholar]
- 19.Keppeke GD, Nunes E, Ferraz ML, Silva EA, Granato C, Chan EK, et al. Longitudinal study of a human drug-induced model of autoantibody to cytoplasmic rods/rings following HCV therapy with ribavirin and interferon-alpha. PLoS One. 2012;7:e45392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novembrino C, Aghemo A, Ferraris Fusarini C, Maiavacca R, Matinato C, Lunghi G, et al. Interferon-ribavirin therapy induces serum antibodies determining ‘rods and rings’ pattern in hepatitis C patients. J Viral Hepat. 2014. July 14. [DOI] [PubMed] [Google Scholar]
- 21.Stinton LM, Myers RP, Coffin CS, Fritzler MJ. Clinical associations and potential novel antigenic targets of autoantibodies directed against rods and rings in chronic hepatitis C infection. BMC Gastroenterol. 2013;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calise SJ, Bizzaro N, Nguyen T, Bassetti D, Porcelli B, Almi P, et al. Anti-rods/rings autoantibody seropositivity does not affect response to telaprevir treatment for chronic hepatitis C infection. Auto Immun Highlights. 2016. December;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Climent J, Morandeira F, Castellote J, Xiol J, Niubo J, Calatayud L, et al. Clinical correlates of the “rods and rings” antinuclear antibody pattern. Autoimmunity. 2016. March;49:102–8. [DOI] [PubMed] [Google Scholar]
- 24.Calise SJ, Carcamo WC, Ceribelli A, Dominguez Y, Satoh M, Chan EKL. Antibodies to Rods and Rings. In: Meroni PL, Gershwin ME, editors. Autoantibodies (Third Edition). San Diego: Elsevier; 2014. p. 161–8. [Google Scholar]
- 25.Feld JJ, Jacobson IM, Sulkowski MS, Poordad F, Tatsch F, Pawlotsky JM. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017. January;37:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017. May 02;166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson DR, Peter J. Hepatitis C virus: how to provide the best treatment with what I have. Liver Int. 2016. January;36 Suppl 1:58–61. [DOI] [PubMed] [Google Scholar]
- 28.Buchner C, Bryant C, Eslami A, Lakos G. Anti-nuclear antibody screening using HEp-2 cells. J Vis Exp. 2014. June 23:e51211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, et al. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015. Front Immunol. 2015;6:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994. December 01;180:2341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh M, Langdon JJ, Hamilton KJ, Richards HB, Panka D, Eisenberg RA, et al. Distinctive immune response patterns of human and murine autoimmune sera to U1 small nuclear ribonucleoprotein C protein. J Clin Invest. 1996. June 01;97:2619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceribelli A, Isailovic N, De Santis M, Generali E, Satoh M, Selmi C. Detection of anti-mitochondrial antibodies by immunoprecipitation in patients with systemic sclerosis. J Immunol Methods. 2017. October 04. [DOI] [PubMed] [Google Scholar]
- 33.Selmi C, Ceribelli A, Gershwin ME. Chapter 57 - Antimitochondrial Antibodies. Autoantibodies (Third Edition). San Diego: Elsevier; 2014. p. 485–90. [Google Scholar]
- 34.Yeaman SJ, Fussey SP, Danner DJ, James OF, Mutimer DJ, Bassendine MF. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988. May 14;1:1067–70. [DOI] [PubMed] [Google Scholar]
- 35.Fregeau DR, Roche TE, Davis PA, Coppel R, Gershwin ME. Primary biliary cirrhosis. Inhibition of pyruvate dehydrogenase complex activity by autoantibodies specific for E1 alpha, a non-lipoic acid containing mitochondrial enzyme. J Immunol. 1990. March 01;144:1671–6. [PubMed] [Google Scholar]
- 36.Calise SJ, Carcamo WC, Krueger C, Yin JD, Purich DL, Chan EKL. Glutamine deprivation initiates reversible assembly of mammalian rods and rings. Cellular and Molecular Life Sciences. 2014. August 2014;71:2963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calise SJ, Purich DL, Nguyen T, Saleem DA, Krueger C, Yin JD, et al. ‘Rod and ring’ formation from IMP dehydrogenase is regulated through the one-carbon metabolic pathway. J Cell Sci. 2016. August 1;129:3042–52. [DOI] [PubMed] [Google Scholar]
- 38.Carcamo WC, Ceribelli A, Calise SJ, Krueger C, Liu C, Daves M, et al. Differential reactivity to IMPDH2 by anti-rods/rings autoantibodies and unresponsiveness to pegylated interferon-alpha/ribavirin therapy in US and Italian HCV patients. J Clin Immunol. 2013. February;33:420–6. [DOI] [PubMed] [Google Scholar]
- 39.Satoh M, Richards HB, Hamilton KJ, Reeves WH. Human anti-nuclear ribonucleoprotein antigen autoimmune sera contain a novel subset of autoantibodies that stabilizes the molecular interaction of U1RNP-C protein with the Sm core proteins. J Immunol. 1997. May 15;158:5017–25. [PubMed] [Google Scholar]
- 40.Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014. January;73:17–23.24126457 [Google Scholar]
- 41.Meroni PL, Biggioggero M, Pierangeli SS, Sheldon J, Zegers I, Borghi MO. Standardization of autoantibody testing: a paradigm for serology in rheumatic diseases. Nat Rev Rheumatol. 2014. January;10:35–43. [DOI] [PubMed] [Google Scholar]
- 42.Chan EK, Fritzler MJ, Wiik A, Andrade LE, Reeves WH, Tincani A, et al. AutoAbSC.Org -- Autoantibody Standardization Committee in 2006. Autoimmun Rev. 2007. September;6:577–80. [DOI] [PubMed] [Google Scholar]
- 43.Shoenfeld Y, Cervera R, Haass M, Kallenberg C, Khamashta M, Meroni P, et al. EASI - The European Autoimmunity Standardisation Initiative: a new initiative that can contribute to agreed diagnostic models of diagnosing autoimmune disorders throughout Europe. Ann N Y Acad Sci. 2007. August;1109:138–44. [DOI] [PubMed] [Google Scholar]
- 44.Muratori P, Efe C, Muratori L, Ozaslan E, Schiano T, Yoshida EM, et al. Clinical implications of antimitochondrial antibody seropositivity in autoimmune hepatitis: a multicentre study. Eur J Gastroenterol Hepatol. 2017. July;29:777–80. [DOI] [PubMed] [Google Scholar]
- 45.Zheng B, Vincent C, Fritzler MJ, Senecal JL, Koenig M, Joyal F. Prevalence of Systemic Sclerosis in Primary Biliary Cholangitis Using the New ACR/EULAR Classification Criteria. J Rheumatol. 2017. January;44:33–9. [DOI] [PubMed] [Google Scholar]
- 46.Dellavance A, Cancado EL, Abrantes-Lemos CP, Harriz M, Marvulle V, Andrade LE. Humoral autoimmune response heterogeneity in the spectrum of primary biliary cirrhosis. Hepatol Int. 2013. June;7:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carcamo W, Yao B, Satoh M, Reeves W, Liu C, Covini G, et al. , editors. Cytoplasmic rings/rods as autoimmune targets of emerging human autoantibodies associated with HCV virus infection and interferon therapy. 9th Dresden Symposium on Autoantibodies. Dresden, Germany: Pabst Science Publishers; 2009. [Google Scholar]
- 48.Calise SJ, Keppeke GD, Andrade LEC, Chan EKL. Anti-rods/rings: a human model of drug-induced autoantibody generation. Frontiers in Immunology. 2015. February 5 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keppeke GD, Calise SJ, Chan EK, Andrade LE. Anti-rods/rings autoantibody generation in hepatitis C patients during interferon-alpha/ribavirin therapy. World J Gastroenterol. 2016. February 14;22:1966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keppeke GD, Prado MS, Nunes E, Perazzio SF, Rodrigues SH, Ferraz ML, et al. Differential capacity of therapeutic drugs to induce Rods/Rings structures in vitro and in vivo and generation of anti-Rods/Rings autoantibodies. Clin Immunol. 2016. December;173:149–56. [DOI] [PubMed] [Google Scholar]


