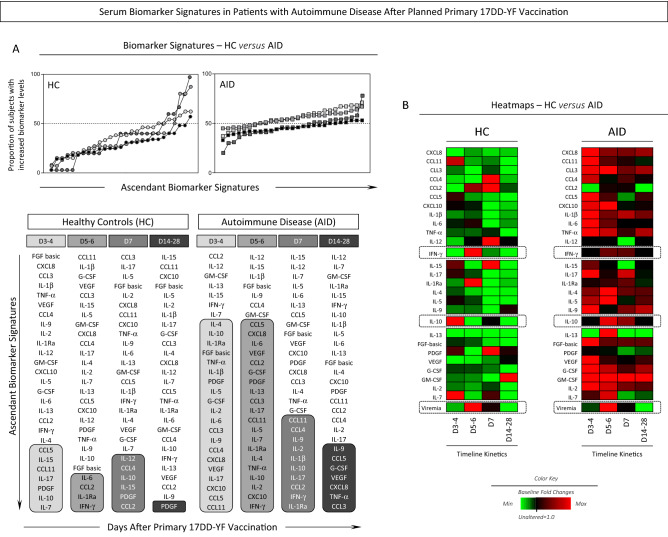
Figure 4.
Serum Biomarker Signatures in Patients with Autoimmune Disease After Planned Primary 17DD-YF Vaccination. (A) Kinetics of serum biomarker signatures at distinct time points after primary 17DD-YF vaccination of Autoimmune Disease patients (AID, n = 140, D3–4 = light grey filled square, D5–6 = grey filled square, D7 = dark grey filled square and D14–28 = black filled circle) and Healthy Controls (HC, n = 21, D3–4 = light grey filled circle, D5–6 = grey filled circle, D7 = dark grey filled circle and D14–28 = black filled circle). The results are expressed as the proportion of subjects with increased biomarker levels (baseline fold change values > 1). Data analysis was carried out considering the 50th percentile as the reference to identify the set of biomarkers with high proportion of subjects with levels above the global median cut-off along the kinetic timeline. Those biomarkers with proportion of subjects with levels above the global median cut-off were highlighted with gray scale rectangles. (B) Heatmaps were constructed considering the baseline fold change values at each time point along the kinetic follow-up (D3–4, D5–6, D7 and D14–28). This approach was employed to draw the overall change in the serum biomarkers profile after primary 17DD-YF vaccination of Autoimmune Disease patients (AID, n = 140) and Healthy Controls (HC, n = 21). Data interpretation was carried out based on the color keys employed to underscore the baseline fold value = 1.0 as the reference for unaltered levels (black filled square), the baseline fold value < 1.0 for decreased levels (green filled square) and the baseline fold value > 1.0 for increased levels (red filled square), according to the paired sample collected at D0.